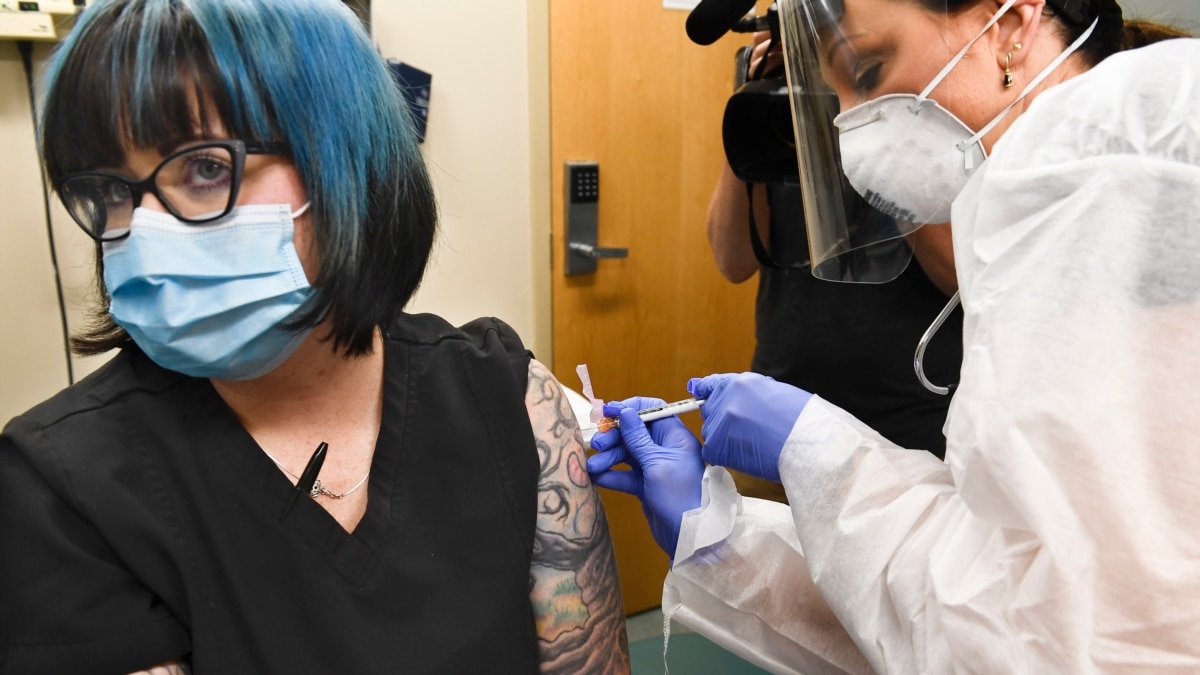
The world’s largest COVID-19 vaccine study began Monday. A total of 30,000 planned volunteers will help evaluate the effectiveness and safety of a vaccine developed by the National Institutes of Health and the drug maker Moderna.
Volunteers will each receive two dose one shot. They will not know if they are receiving the real vaccine or a fake version. Then, the scientists will closely follow the volunteers as they go about their daily activities. They want to see which group experiences a higher infection rate, especially in areas where the virus is still spreading uncontrollably.
There is still no guarantee that the experimental vaccine will offer protection. The study hopes to answer that question.
Dr. Anthony Fauci is the country’s leading infectious disease expert with NIH. He told the Associated Press: “Unfortunately for the United States of America, we have a lot of infections right now” to help get that answer.
Volunteers from more than 80 test areas across the country will participate in the study. Moderna said the first vaccines were administered Monday morning in Savannah, Georgia.
Several other vaccine candidates made by China and the Oxford University of Great Britain started later.stage studies earlier this month. Those studies involved fewer volunteers than Americans. They are being carried out in Brazil and other affected countries.
The United States government requires its own tests of any vaccine that can be used domestically. The goal is not just to test whether a vaccine works. It is also to check if it is safe.
Through the government-funded COVID-19 Prevention Network, the United States plans a new study for vaccine candidates every month through the fall. Each will involve 30,000 newly elected volunteers. The hope is that, by using the same rules for each study, scientists can easily compare the vaccines.
In August, the United States will complete its final stages of the Oxford University vaccine candidate. The Johnson & Johnson vaccine study will begin in September and the Novavax study in October. Drug maker Pfizer also plans to conduct its own study of 30,000 people this summer.
Many volunteers are needed to test possible vaccines. But in the past few weeks, more than 150,000 Americans have signed up to volunteer for the studies, says Dr. Larry Corey. He is with the Fred Hutchinson Cancer Research Institute in Seattle, Washington.
“These tests … need reflect the diversity of the United States population, “Corey said at a vaccine meeting last week.
He said it is important that the studies include people from different age groups and from populations that have been especially affected by COVID-19, including blacks and Hispanics.
It usually takes years to create a new vaccine from start to finish. But this time, scientists are setting speed records. They believe that vaccination is the world’s best hope against the virus.
The new coronavirus was not even known to exist before the end of December. Vaccine makers went to work on January 10, when China shared the genetic sequence of the virus.
Just 65 days later, US researchers administered the first test vaccine made by the NIH and Moderna to Jennifer Haller, a volunteer in Seattle, Washington. Haller is urging others to volunteer now.
She told AP: “We all feel so helpless right now. There is very little we can do to combat this virus. And being able to participate in this test has given me the feeling that I am doing something. “
That early study included Haller and 44 others. It showed that the experimental COVID-19 vaccine produced antibodies in a small group of healthy people. It caused some minor side effects, such as a brief fever, cold and pain in the injection area. Early tests of other leading vaccine candidates have had equally promising results.
It will take several months for the first data to arrive from the test of 30,000 Moderna volunteers, followed by the Oxford test.
Until then, Haller will continue to wear a mask in public. She still follows the same recommended physical distance for everyone. “I don’t know what the chances are that this is the right vaccine,” he said. “But thank God there are so many others fighting this right now.”
I’m Ashley Thompson
Hai Do adapted this story to learn English from an Associated Press news report. Ashley Thompson was the editor.
_______________________________________________________________
Words in this story
dose – n. the amount of medicine taken at the same time
stage – n. a particular period in the development of something
reflect – v. to show something
fever – n. a body temperature that is higher than normal
cold – n. a cold feeling
.


