[ad_1]
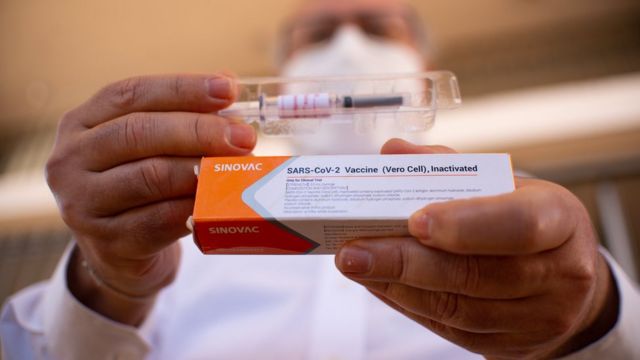
Image source, fake images
The Corona Wac trial was conducted with large groups of volunteers in Brazil, Indonesia and Turkey.
Brazilian authorities suspend clinical trials to test the efficacy of the COVID-19 vaccine. The main character of China is temporarily inactive. After the “Serious Adverse Reactions from Drug Use” report was found
Anvisa, Brazil’s national health regulator, said: The incident occurred on October 29. But he did not elaborate.
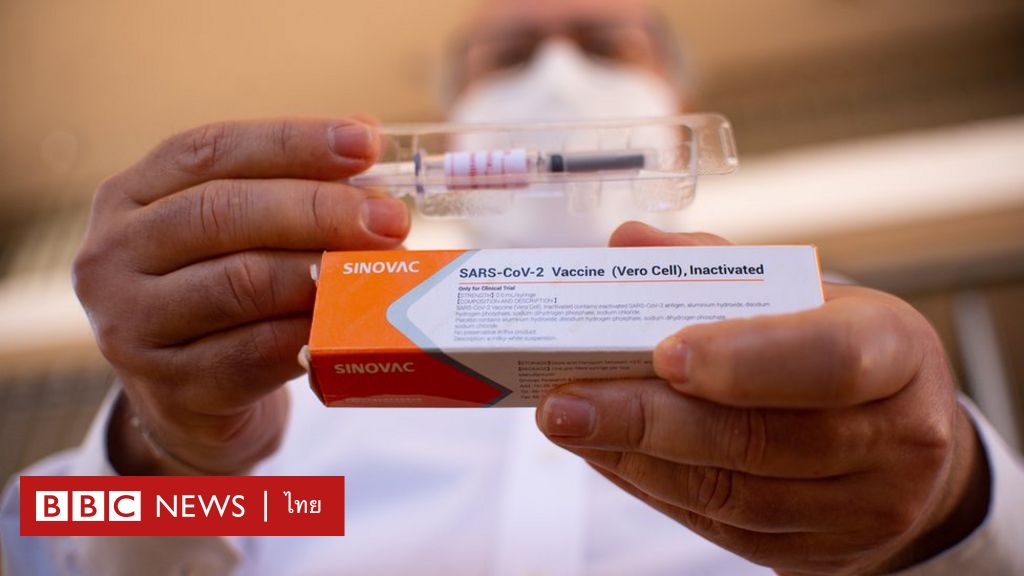
The vaccine that Brazilian authorities have suspended testing is CoronaVac, developed by the Chinese biopharmaceutical company Sinovac Biotech. It is one of the new coronavirus vaccines in the final trial. And it’s being watched from all over the world
Sinovac Biotech stated that the company “Guarantee the safety of this vaccine”
Sinovac Biotech has used the CoronaVac vaccine to immunize many Chinese citizens in mainland China under an emergency program to control COVID-19.
Brazil is one of the largest new coronavirus outbreaks in the world. The cumulative figure of more than 5.6 million confirmed people have been infected and the third-highest death toll in the world after the United States and India is nearly 163,000, according to Johns Hopkins University.
What happened
Anvisa announced yesterday (November 9) the suspension of clinical trials testing the efficacy of the “Coronavac” vaccine after experiencing “serious adverse drug reactions.” What happened OR where the incident happened
Dimascovas, director of the Butantan Institute, which conducts coronavirus vaccine trials in Brazil, told local media: The suspension of the trial was related to the death of participating volunteers, however, it was confirmed that the deaths were not directly related with the vaccine itself.
Sinovac Biotech said today (November 10) that it was in talks with Brazilian authorities about the case.
“We were informed by the director of the Butanton Institute. It is believed that serious adverse drug reactions were not related to vaccines … Clinical trials in Brazil are strictly conducted. By adhering to the principles of Good Clinical Practice (GCP), we are confident in the safety of this vaccine, ”the company said.
In addition to Brazil There are still clinical trials to test the efficacy of Coronavac in Indonesia and Turkey, but no publications have been announced in the two countries.
Reuters reported that the official Indonesian company Biopharma. Today revealed that Corona Wac’s national clinical trial “Goes Smoothly”
When will COVID-19 be available?
Is it normal to suspend vaccine trials?
Suspension of clinical trials for vaccines is not uncommon. In September, the UK suspended final testing of the COVID-19 vaccine. This is a collaboration between the University of Oxford and Astra Seneca Company. (AstraZeneca) after a participant had an adverse drug reaction.
However, the trial started again a few days later. Once the regulator determined that it was safe to continue the experiment.
Brazilian President Jair Bolsonaro said he was more interested in the vaccines developed by the University of Oxford and Astra Seneca, and said his government would. Don’t buy a vaccine made in China.
Investigating the COVID-19 vaccine Where is it already
Coronavac is one of dozens of vaccines worldwide in the final trial. Also known as the Phase III trial, it is an important step in the development of a vaccine. And some vaccines may not be tested.
The news of the suspension of the trials in Brazil came to light. After Pfizer in the United States announced that initial analysis indicates that the first COVID-19 vaccine can prevent up to 90% of people from contracting the new coronavirus.
How does China use an experimental vaccine?
In addition to the third stage of trials abroad, China also offers a vaccine against COVID-19. Which is still in the process of testing for people in the country too
Coronavac is one of three still experimental vaccines that Chinese authorities have injected into hundreds of thousands of people in an emergency program to control COVID-19.
Last month the BBC News team recorded hundreds of people queuing to get vaccinated in Yiwu. In Zhejiang Province After the government has approved vaccination for all people who need it.
A businessman who was about to receive the required second dose told the BBC that he would definitely be vaccinated. Noting that the high rates of contagion abroad are worth considering.
Sinovac Biotech previously said that most of its employees and family members had received the coronavirus vaccine. A Chinese health worker said that no serious side effects were observed when monitoring clinical trials of the vaccine.