
Electric cars have come a long way in terms of to go a long way on a load. But everyone is still looking for the next big leap in battery technology – a battery with significantly higher energy density would mean more range than lower cost to reach the current range. There is always some room for incremental advances in today’s lithium-ion battery technology, but there’s a lithium-holy grail that has been out of reach for decades: its graphite anode unlocked to shrink the cell.
A lithium metal battery would simply use solid lithium as the anode instead of a graphite frame needed for lithium atoms to pack when the battery is charging. The problem is that lithium does not form a charge surface during charging, so the battery capacity drops drastically – in some configurations it drops to 80 percent in 20 configurations. Rogue lithium also tends to build up dangerous, smart, needle-like structures that can break through the separation between the anode and cathode and short-circuit the cell.
Last year, a lab group from Dalhousie University with ties to Tesla developed a lithium metal battery with slightly better performance. Lithium atoms electroplate on a copper electrode as the battery charges and then charge back into a conventional lithium-nickel-manganese-cobalt cathode as charge depot. With a new electrolyte, they were able to charge this battery for about 90 cycles before hitting 80 percent capacity to deal with the nasty short-circuit problem.
In a new study, the team reports on a post-mortem of that design that identifies the causes of capacity loss. As a result, they find a tweak that throws them up to about 200 cycles.

This battery would be a major advancement when it comes to viability, and holds about 60 percent more energy per volume per unit than lithium-ion batteries used today. That could increase the range of electric cars from 400 kilometers to 680 kilometers (or 250 miles to 400), the researchers note. The improved stability is due to an electrolyte composed of two lithium boron fluorine salts in an organic solvent. To see what happened in the battery, the team analyzed changes in the electrolyte over time and also tracked changes in the behavior of the solid lithium formation on the anode.
The salts in the electrolyte, it turns out, were consumed when the battery went through charge cycles. Reactions on the cathode side convert one of the salts into another, but reactions on the anode side consume both salts without regenerating. This is because the battery went through more and more cycles, there was less of the electrolyte available to do its job.
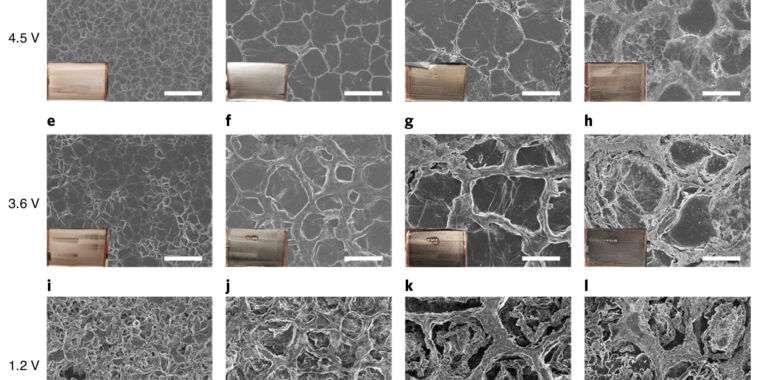
Throwing the anode edge under an electron microscope revealed that the cyclic plating and removal of solid lithium became less and less orderly over repeated cycles. It begins with the formation of a very smooth layer, but develops topography through the fiftieth cycle or so. Pockets form between ring-like walls, leading to increasing portions of lithium that are electrically insulated – and no longer play along with the full and heavy thump of the battery. (See the image at the top of this page.) This also means that the surface of the solid lithium grows, so more electrolyte would be needed to maintain contact everywhere.
One possible solution would be to increase the volume of electrolyte so that it takes longer for the salt to disappear, as well as to maintain better contact over the entire surface. However, this would reduce the energy density of the battery, so the team decided to dissolve the concentration of the salt dissolved in the electrolyte.
As the concentration rose, the battery was able to maintain its capacity for more charge cycles – hitting at least 150 cycles before dropping to 80 percent. Or to put it another way, it has taken about 200 cycles to fall to the equivalent capacity of a lithium-ion battery of that size. Another thing that helps is actually some simple clamping pressure, as this encourages the solid lithium to pack better together. All of these numbers come from batteries that are placed in a small checkerboard. Without that pressure, capacity drops faster and faster.
These results show significant progress on an idiosyncratic problem, but this design has a way of going to meet the longevity of current lithium-ion batteries, which can peak 1,000 cycles before decreasing to that 80 percent mark . The researchers write, “Nevertheless, increased lifespan is required before such cells are viable for electric cars such as electrified urban aviation. If further advances in life can be achieved, anode-free lithium metal cells with liquid electrolytes present the most straightforward and inexpensive pathway to lithium life. high energy density. ”
Natural Energy, 2020. DOI: 10.1038 / s41560-020-0668-8 (About DOIs).