[ad_1]

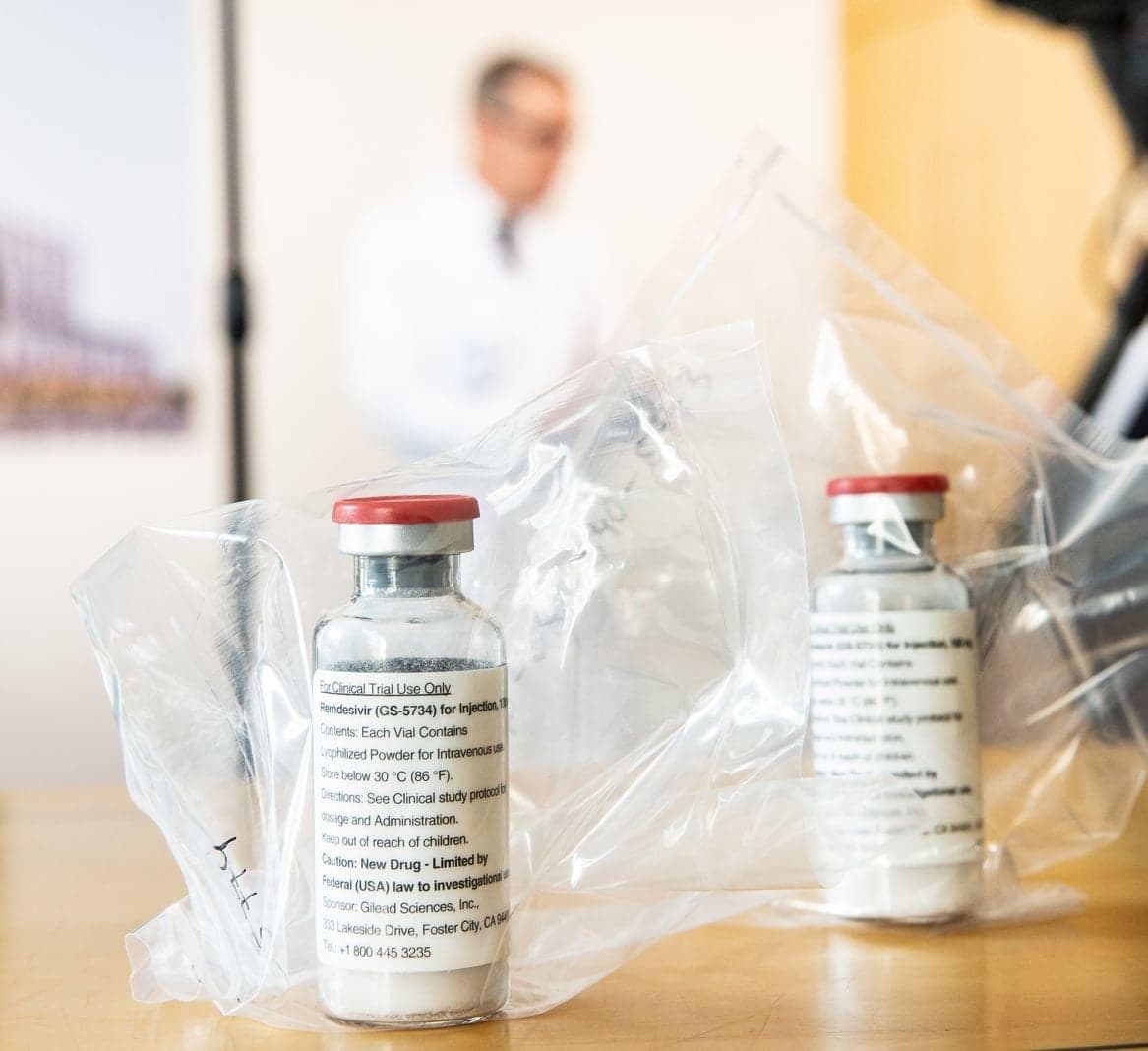
Remdesivir is a beacon of hope in the search for agents for the treatment of Covid-19, studies on the effectiveness of the substance started early. The first results of China and the USA. USA They are now available, but these are in clear contradiction to each other.
The active ingredient remdesivir may shorten the duration of treatment in patients with Covid 19, according to a US study. USA The study results are very positive, said the immunologist and head of the United States National Institute of Infectious Diseases (NIAID), Anthony Fauci, on Wednesday (local time).
A Chinese study presented in the journal “The Lancet”, on the other hand, concludes that the condition of patients with remdesivir does not improve significantly. However, due to the lack of patients, this study was discontinued early.
Remdesivir: Crowd over corona medication, but first signs of hope
According to Fauci, an adviser to the President of the United States, Donald Trump, remdesivir has shown “a significant positive impact in reducing recovery time.” The clinical study with more than 1000 participants was carried out with control groups, and the data collection was accompanied by independent experts. However, the results would still have to be independently verified and published. In any case, the indications for a significantly shorter duration of the disease are promising.
According to Fauci, patients with Covid-19 lung disease who received remdesivir in hospitals recovered after an average of 11 days, and patients in the control group only after 15 days. However, this does not solve all problems, Fauci said during a meeting in President Trump’s office. The mortality rate was also somewhat lower, but this result has not yet been statistically significant.
Coronavirus: 20 quotes from Donald Trump’s Fremdschämen on Covid-19
The Chinese study included 237 patients from ten hospitals in Wuhan, the site of the pandemic. 158 received remdesivir, 79 an ineffective fake drug. The researchers found no statistically significant influence on disease duration or death rate. However, in addition to the early termination of the study, they point to another weakness of the study: Most of their patients were treated with Remdesivir only quite late in the course of the disease. Starting therapy earlier can improve treatment results.
British medical statistician John Norrie of the University of Edinburgh stressed in a comment accompanying the study that high-quality research into active ingredients that promised initial attempts at treatment should not be given up. “This is a particular challenge in the midst of a pandemic, the temptation to lower the threshold of compelling evidence is great.” The use of ineffective and potentially dangerous measures must be avoided, because it is more a pity than the use and complicates clinical studies on really effective means. .
Does an herbal tea help against the corona virus? Students have to take it
According to German experts, the results of the United States study are reliable enough. Sufficient patients have been examined; Clemens Wendtner of the Schwabing Clinic in Munich said they had been discharged from the hospital on Remdesivir therapy. “This means that the study’s essential endpoints have been reached, so there should be little doubt in my opinion that the substance will be approved quickly.”
Gerd Fätkenheuer of the Cologne University Hospital, head of a clinical trial of Remdesivir (GS-5734) in patients in Germany, awaits approval soon due to the positive results.
Remdesivir was originally developed against the Ebola virus and has shown some efficacy against Sars-CoV-2 in laboratory tests. In cell experiments, it prevented the virus from multiplying, in animal experiments it worked against other coronavirus infections like Sars and Mers. Remdesivir has not yet been approved in any country in the world.
Warn the scientists! Celebrity “tips” are so dangerous
The Food and Drug Administration (FDA) is currently in talks with drug maker Gilead to quickly make remdesividos available to hospital patients, Fauci said. However, formal approval of the drug takes much longer and requires more study.
Trump said Wednesday night (local time) when asked if he wanted the agency to expedite the drug approval process, he wanted to get it up and running as soon as possible. “We want very fast approvals.”
Biotech company Gilead said in a press release that they were aware of the “positive data” from the clinical trial, but that the communication was with NIAID.
Source: dpa