[ad_1]
Pharmaceutical companies around the world are racing to develop COVID-19 vaccines.
Reuters reported that, as of August, there were more than 200 vaccine candidates in development globally, including more than 20 in human trials.
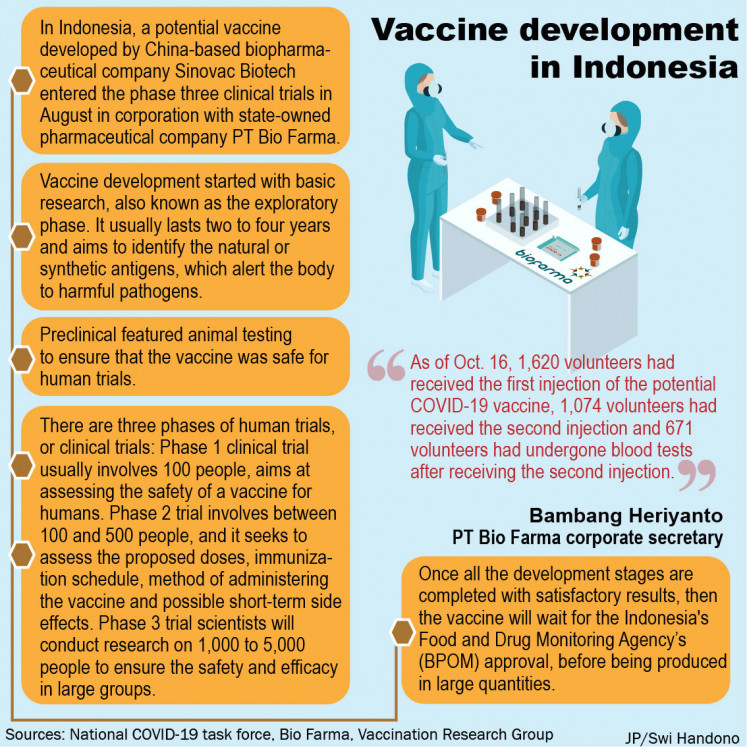
In Indonesia, a potential vaccine developed by China-based biopharmaceutical company Sinovac Biotech entered phase three of clinical trials in August.
Bambang Heriyanto, corporate secretary of the state pharmaceutical holding PT Bio Farma, said on Monday that, as of October 16, 1,620 volunteers had received the first injection of the possible COVID-19 vaccine, 1,074 volunteers had received the second injection, and 671 volunteers had underwent blood tests after receiving the second injection.
 Vaccine Development in Indonesia (JP / Swi Handono)
Vaccine Development in Indonesia (JP / Swi Handono)
With all eyes on the COVID-19 vaccine, the coordinator of the COVID-19 national task force team of experts and spokesperson Wiku Adisasmito tried to explain the stages of vaccine development.
Wiku said Thursday that development of the vaccine began with basic research, also known as the exploratory phase.
The stage features basic laboratory research that focuses on examining the virus and the cells associated with it.
According to Vaccination Research Group (VRG), this phase usually lasts two to four years and aims to identify natural or synthetic antigens, which alert the body to harmful pathogens.
Wiku explained that some potential vaccines were also being prepared in the exploratory phase and would be followed by the preclinical phase.
The preclinical stage included animal testing to ensure the vaccine was safe for human trials, Wiku said.
There are three phases of human trials or clinical trials.
Also read: Bio Farma will produce more than 16 million doses of COVID-19 vaccine per month
The phase 1 clinical trial generally involves 100 people. Wiku explained that this phase was aimed at evaluating the safety of a vaccine for humans.
The phase II clinical trial will involve between 100 and 500 people, and seeks to evaluate the proposed doses, the vaccination schedule, the method of administration of the vaccine, and possible short-term side effects.
The phase III clinical trial will follow. In this phase, scientists will conduct research on 1,000 to 5,000 people to ensure safety and efficacy in large groups.
Once all stages of development are completed with satisfactory results, the vaccine will await approval from the Indonesian Food and Drug Monitoring Agency (BPOM), before being produced in large quantities.
Earlier this week, Wiku explained that the COVID-19 vaccine would be available once all clinical trials showed it to be safe and effective.
However, Wiku said that COVID-19 vaccines were not the only guarantee for the country to end the pandemic, explaining that the vaccines only served as a form of medical intervention to boost people’s immunity during the pandemic. Wiku urged the public to comply with the “3M” health protocol of mask use, hand washing and physical distancing, while waiting for the COVID-19 vaccine. (jes)
Editor’s note: This article is part of a public campaign by the COVID-19 task force to raise awareness about the pandemic.
Your premium period will be expires in 0 day (s)
close x

Subscribe for unlimited access 50% off now
[ad_2]