[ad_1]
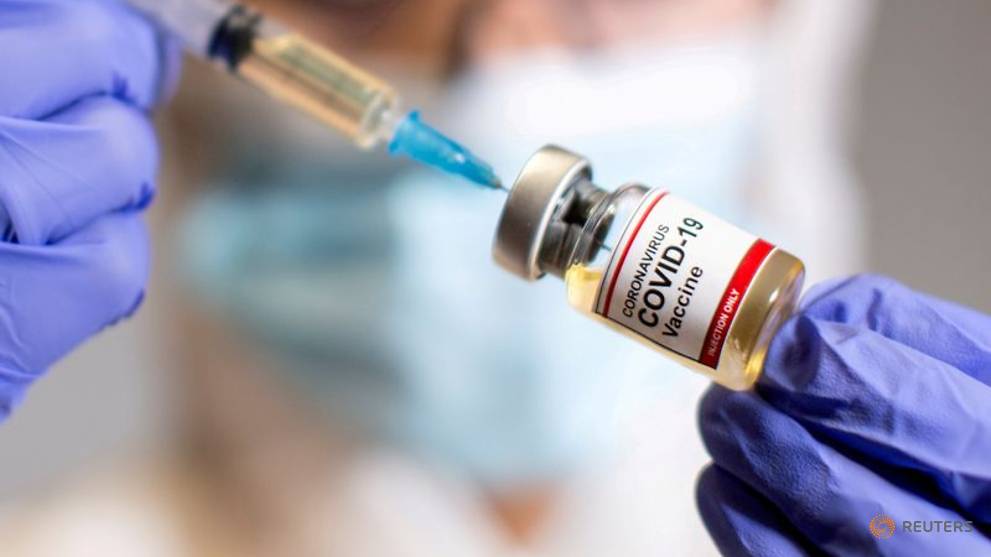
PARIS: Britain became the first Western country on Wednesday (December 2) to approve a COVID-19 vaccine for general use, giving the green light to the Pfizer-BioNTech drug.
As authorities in the US and Europe investigate other candidates, this is how countries have accelerated their approval procedures to deal with the pandemic.
GREAT BRITAIN: “ROLLING COMMENTS”
Britain was able to approve the Pfizer vaccine after the Medicines and Healthcare Products Regulatory Agency (MHRA) gave its go-ahead.
The MHRA used a “continuous review” process since June to evaluate the vaccine in record time.
Teams of scientists had worked “around the clock” on different aspects of the security assessment, often tackling several issues in parallel, MHRA chief June Raine said.
READ: UK approves Pfizer-BioNTech vaccine for use, world’s first
Health Secretary Matt Hancock and others claimed Britain’s departure from the EU had allowed it to pass the vaccine faster than its continental neighbors.
“Unlike the EMA (European Medicines Agency), they can ask questions on the fly and get answers faster as a single agency,” said Penny Ward, professor of pharmaceutical medicine at King’s College London.
Raine insisted that “no corners have been cut at all” in the vaccine approval process.
Jonathan Van-Tam, England’s deputy chief medical officer, told the BBC on Thursday that “I don’t really expect other regulators … to be very behind on this vaccine,” and said approvals elsewhere would probably be “a matter of days”. “.
Some other countries are also adopting an ongoing review process, which allows companies to submit real-time data from ongoing vaccine studies while the regulatory review is taking place simultaneously.
Singapore, for example, is evaluating initial data on a COVID-19 vaccine presented by Moderna.
“This pathway could speed up the approval process and, at the same time, ensure that there is adequate scientific evidence to support the quality, safety and efficacy of the product,” said the country’s Health Sciences Authority.
READ: HSA Evaluates Initial COVID-19 Vaccine Data Submitted by Moderna for Use in Singapore
EU: ACCELERATED PROCEDURES
The Amsterdam-based EMA, which regulates medicines in all 27 EU countries, has also resorted to a process of “continuous review” of safety and efficacy data from the developers of the COVID-19 vaccine.
The three most advanced candidates, Pfizer / BioNTech, Moderna and Oxford / Astrazeneca, have already been subject to the scheme for several weeks.
According to the EMA, expedited procedures are granted for drugs “that meet an unmet medical need based on less complete data than is normally required.”
READ: EU criticizes UK’s ‘hasty’ approval of COVID-19 vaccine
The normal pipeline of vaccines would see all the data collected and presented at the beginning of the authorization process.
An EMA decision on the Pfizer-BioNTech vaccine is expected “no later than December 29,” while a decision on Moderna’s version should follow by January 12.
It is up to the European Commission in Brussels to give the final green light.
US: ADVISORY COMMITTEE
Both Pfizer-BioNTech and Moderna have applied for an Emergency Use Authorization (USA) for their COVID-19 vaccines from the US Food and Drug Administration (FDA).
But the American process is slower than the British one and involves a public consultation.
The FDA conducts its own review of the vaccine and requests an independent advisory committee.
READ: US hopes to vaccinate 100 million people against COVID-19 by February
“The FDA process is a completely transparent process with independent experts commenting and asking questions and recommending or advising the agency,” Moncef Slaoui, scientific advisor to Washington’s Operation Warp Speed (OWS) program, said Wednesday.
The advisory committee is scheduled for a meeting on December 10 on the Pfizer-BioNTech vaccine and for Moderna on December 17.
FDA decisions on the two drugs must follow those meetings. If approved by the agency, the vaccines could be available in the United States, the world’s worst-hit country with more than 270,000 deaths, in December.
RUSSIA: SIMPLIFIED PROCEDURE
The “scientific center for drug evaluation” of the Russian Ministry of Health conducts tests on medical products under development.
According to its official vaccine website, “unlike in many countries, there is a state testing system, using comparable drugs, a double-blind study, and other developer-independent control tests.”
President Vladimir Putin ordered the government to simplify procedures for state registration of some drugs, in order to speed up the approval of a vaccine.
READ: Putin orders Russia to start with massive COVID-19 vaccines
Trials of the country’s Sputnik V vaccine began in mid-February, and the first and second phases of clinical trials were completed on August 1.
Authorities approved the inoculation on August 11 before Phase 3 trials began. The large-scale study of 40,000 volunteers is now complete, but the results have not been made public.
Putin has told authorities to begin “large-scale” vaccinations among at-risk populations starting next week.
The drugs should be available to the Russian public in early 2021.
Russia has also started a mass vaccination campaign in the army, hoping to vaccinate 80,000 soldiers by the end of this year and more than 400,000 servicemen in the future.
CHINA: LAST STAGE TESTING AND EMERGENCY USE
China has four coronavirus vaccines in phase 3 trials, usually the last step before regulatory approval, but has not released much of its data.
READ: Alibaba’s Cainiao Says He’s In Talks With Chinese Vaccine Manufacturers About COVID-19 Logistics
Although regulators have yet to approve China’s vaccines for mass distribution, the country has approved some advanced candidates for emergency use, hitting people ranging from state employees to international students since July.
Nearly a million people have already received an experimental Sinopharm vaccine, the company said in November. Another company, Sinovac Biotech, previously said that nearly all of its employees and their families had been voluntarily vaccinated.
CHECK THIS: Our comprehensive coverage of the coronavirus outbreak and its developments
Download our app or subscribe to our Telegram channel for the latest updates on the coronavirus outbreak: https://cna.asia/telegram