[ad_1]
One of the world’s fastest efforts to develop a Covid-19 vaccine is lagging behind its rivals, its progress appearing to be hampered by political tensions between China and Canada and concerns that its injection may not work as well as others.
CanSino Biologics Inc., the Chinese company that began the world’s first human trials with an experimental coronavirus vaccine in March, has yet to begin critical trials on the final stage of the vaccine it developed with the Chinese military. Meanwhile, rivals like the Americans Moderna Inc. and Great Britain AstraZeneca Plc and China Sinovac Biotech Inc. and Sinopharm is in this final phase of testing, administering its vaccines to thousands of people to see if they work.
With its Phase III trials yet to begin, CanSino hasn’t had a chance to allay concerns from the previous stage data, which showed the immune response generated by its injection. it varied widely among participants. Its setbacks offer a glimpse into the scientific and political uncertainties companies face as they compete to produce a vaccine against the virus that has already killed more than 850,000 people around the world.
Just a few months ago, the Tianjin-based biotech firm positioned itself at the forefront of global vaccine trials thanks to a partnership with the Canadian government’s main research agency, which enabled the company to conduct tests in the North American country. CanSino was supposed to ship its vaccine candidate, Ad5-nCoV, developed with Canadian technology, to Canada so that end-stage testing could begin there as early as the fall. The vials never arrived.
Chinese customs has not approved shipments of the CanSino vaccine to Canada, the National Research Council of Canada said in an Aug. 26 email. The incident appears to be part of a pattern of retaliation against Canada since it arrested Meng Wanzhou, chief financial officer of the Chinese telecommunications giant. Huawei Technologies Co., in a US delivery request in December 2018. In recent months, the relationship between the two countries has only worsened.

Photographer: Darryl Dyck / Bloomberg
Guy Saint-Jacques, a former Canadian ambassador to China, said it is clear that blocking the CanSino vaccine to Canada is not just a bureaucratic glitch because the company appears to have shipped shipments to countries that are friendly to China.
“This is part of China’s Covid-19 diplomacy,” he said. “Unfortunately, it is part of the general difficulties that we are having with China.”
Global ties
For CanSino, international collaboration is vital because late-stage trials require large-scale testing at the site of an active outbreak, something that is no longer possible within China, which has largely eliminated local transmission. The company, in response to questions, pointed to recent stock market filings and declined to comment further.
In a presentation by the Hong Kong Stock Exchange, he said that he had not started registering participants for Phase III trials as of Aug. 18. In another statement on Aug. 27, he said that the collaboration between the National Research Council of Canada and the company wasn’t over.
Since Meng’s arrest, China has jailed two Canadians on espionage charges, stopped billions of dollars in Canadian imports and put four other Canadians on death row. In addition to relations between Beijing and Ottawa, Canada also suspended its extradition treaty with Hong Kong in response to a new security law imposed there by China.
China’s General Administration of Customs did not respond to a request for comment. In its email, the National Research Council of Canada said that while the CanSino-Canada partnership had previously been reviewed by the Chinese government, after it was signed, Beijing made changes regarding the export of vaccines. Canada was ready to begin preliminary trials in June but, due to the delay, the research council is focusing on other partners, he said. Canadian Prime Minister Justin Trudeau described the advances in the CanSino vaccine as “unfortunate.”
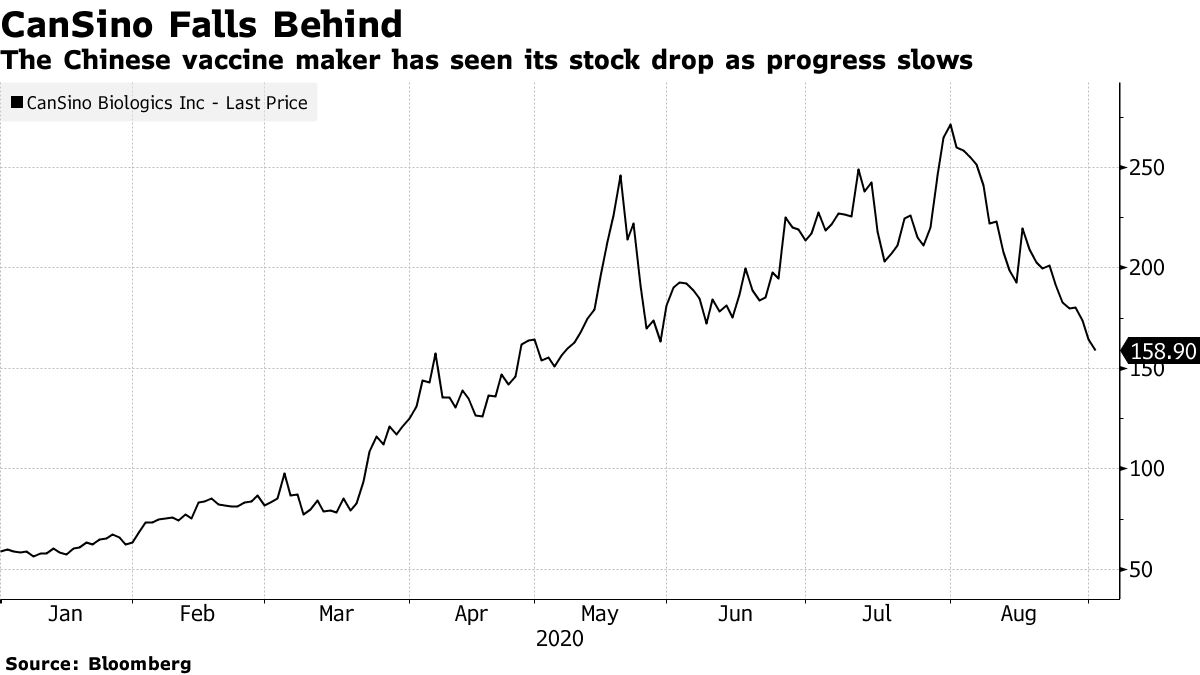
After hitting an all-time high of HK $ 271.4 on July 31, CanSino’s stock fell steadily in August, losing nearly 40%, when news emerged that the vials it was supposed to ship to Canada for its test never left China. The stock lost as much as 11% on Wednesday, but is still up 140% since the beginning of this year.
CanSino, meanwhile, has had other struggles. Since most of the vaccine pioneers have now published their first human test data, there have been some concerns that antibodies activated by CanSino injection as part of a vaccine-induced immune response may be mediocre in comparison. with those stimulated by rivals, said Brad Loncar. CEO of Loncar Investments in the USA
That could be the reason the company has seemed slow in closing deals with countries to conduct phase III trials, said Loncar, who has stakes in the Chinese vaccine developer.
“Of all the data I’ve seen from companies that have published data on the human stage, I would say that CanSino is one of the ones that would make me the most nervous about Phase III,” Loncar said. “Antibody levels in general, when you compare them to what has been published by other companies, such as Moderna and Pfizer / BioNTech and even with other Chinese vaccine manufacturers such as Sinovac and Sinopharm, I think the data has not validated the CanSino data. position as leader “.
While analysts have celebrated Based on the different standards used to measure the vaccine-induced immune response by different groups, CanSino suffers from a particular challenge: its vaccine uses a genetically modified human adenovirus, which causes the common cold and to which many people already have immunity. That pre-existing immunity has been shown to weaken the vaccine’s ability to generate the kind of antibodies that can bind to spikes on the surface of the coronavirus to prevent it from entering human cells.
Russian trials

A nurse shows a Covid-19 vaccine produced by Sinovac Biotech, in Brazil, on August 8.
Photographer: Silvio Avila / AFP via Getty Images
Meanwhile, other Chinese vaccine manufacturers have been more successful in phase III trials. Beijing-based Sinovac has begun vaccinating people in Brazil and Indonesia, while more countries are signing until be part of the company multi-Last stage test center. State owned China National Biotec Group is testing the two candidate vaccines it developed in the United Arab Emirates and has obtained approval for further testing in Peru, Argentina and Morocco.
For more, read: China’s vaccine leader aims to beat Covid the old-fashioned way
As for CanSino, in addition to the delayed trial in Canada, one of the company’s founders, Qiu Dongxu, said in a forum in July that the company is speaking with Russia, Brazil, Chile and Saudi Arabia for the final stage of testing. Of these, only Russia appears to have materialized so far, and local pharmaceutical company PetroVax agreed to conduct a small trial in which the 625 participants will not receive the injection until the end of September.
A large-scale phase III seeking to enroll 40,000 people is planned for Pakistan, according to an online clinical trial database. The trial has not yet begun and will be led by researchers from the China Center for Disease Control and Prevention and the Canadian Center for Vaccination.
The CanSino vaccine still deserves more testing, as it is not yet known how strong the immune response the vaccine can underpin if given twice, a strategy that has been tested by almost all pioneers except CanSino. Trials in Canada could uncover the effect of a booster vaccine, if the vials ever reach Canadian shores.
– With the assistance of Natalie Obiko Pearson and Yuliya Fedorinova
(Updates to the share price in paragraph 12)