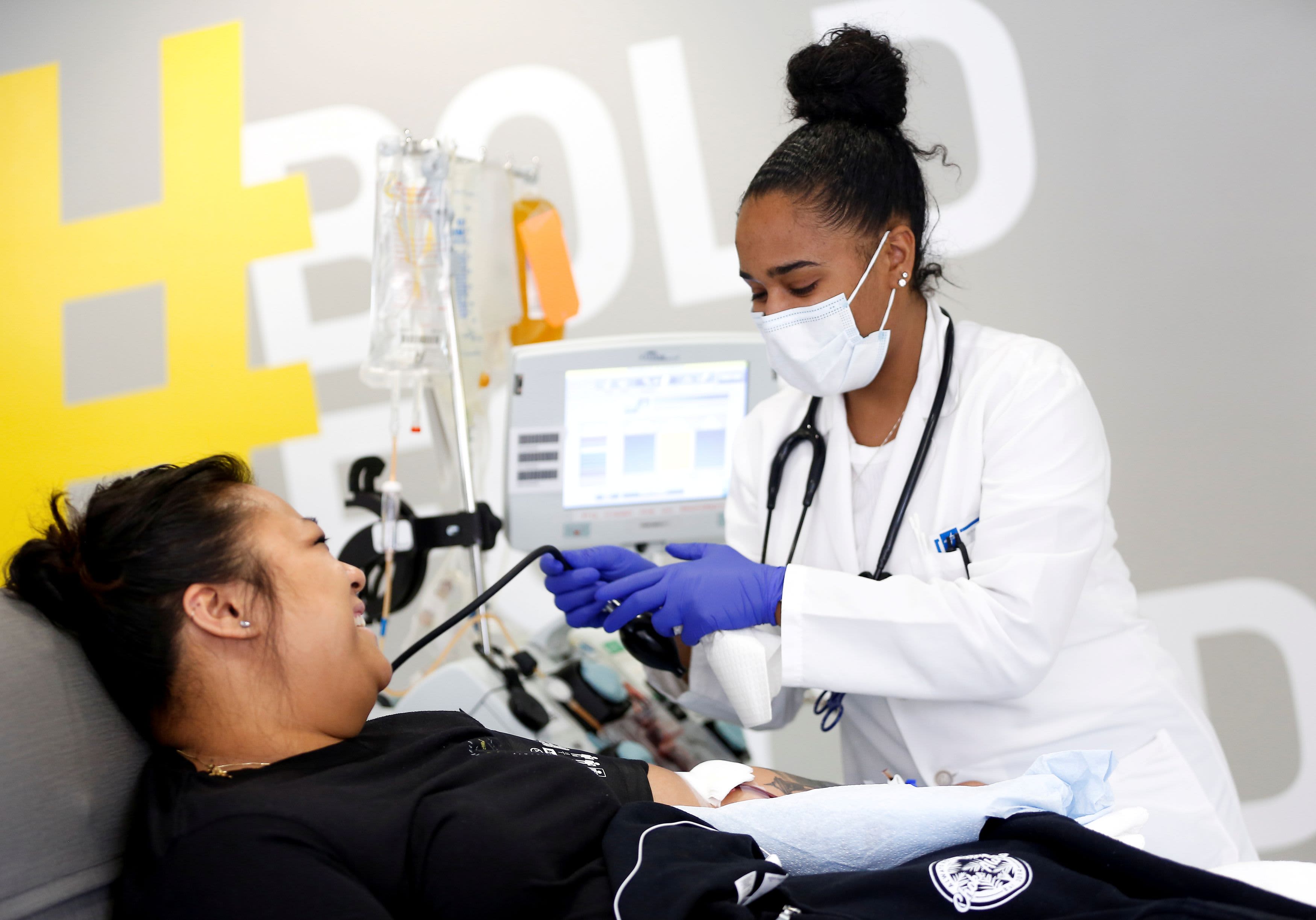
Scientists and public health officials said Monday they are skeptical overpowering plasma is an effective treatment for patients hospitalized with Covid-19, even after the Food and Drug Administration granted an emergency use authorization for the treatment and President Donald Trump showed it as a breakthrough. “
There are no formally approved medicines or vaccines for coronavirus. Convalescent plasma is one of several therapies that are being tested as a potential treatment.
Plasma, taken from patients recovering from Covid-19 and developing antibodies against the virus, is injected into sick patients. Scientists hope it will help start the immune system in fighting the virus. A study of 35,000 patients posted earlier this month by the Mayo Clinic and sponsored by the National Institutes of Health suggested that plasma may reduce mortality in some patients who are in the hospital. Although the study did not have a placebo group to compare the results, it became difficult to determine whether the treatment actually worked.
The FDA said Sunday that it provided emergency use that would allow health care providers in the U.S. to treat suspected or laboratory-confirmed Covid-19 in hospital patients with the disease. The agency said it was reasonable to believe that the treatment could be effective in treating Covid-19 patients, and the known and potential benefits outweigh the known and potential risks.
Adequate and well-controlled randomized trials “remain necessary” for a definitive demonstration of efficacy and “to determine the optimal product attributes and appropriate patient populations for their use,” the FDA said in allocating emergency use.
“This is a powerful therapy that transfuses very, very strong antibodies from the blood of recovering patients to help patients fight an infection. It has an incredible rate of success,” Trump said Sunday night in a White House letter. , and called the treatment a “breakthrough.” “Today’s action will dramatically expand access to this treatment.”
Scientists and public health officials have doubts, and said more data from randomized controlled trials, which are considered the “gold standard” in science, is still needed to know if it is safe and effective. Results of threads were not conclusive, were relatively small and provided “very low quality evidence”, according to the World Health Organization.
“We followed this up,” said Drs. Soumya Swaminathan, the chief scientist of the WHO, in Geneva on Monday. “We are conducting ongoing meta-analyzes and systematic reviews to see where the evidence is shifting or pointing, and at present it is still evidence of low quality. That we recommend that convalescent plasma is still an experimental therapy. be -designed, randomized, clinical trials. “
She said countries could use it on an emergency basis “if they feel the benefits outweigh the risks, but that is usually done while they wait for the definitive evidence, which is yet to come.”
The FDA has issued emergency use authorization for various tests for coronavirus and some medications. In May, the antiviral inhibitor agency granted the authorization, allowing hospitals and doctors to use the drug on hospitalized Covid-19 patients. Hydroxychloroquine was also granted emergency authorization, but the FDA later removed the designation once the agency found that the malaria drug was likely to be effective.
Results of the study by the Mayo Clinic that the FDA cites in its authorization for plasma treatment indicate that patients under the age of 80 who were not on a respirator and received plasma with a high level of antibodies within three days of diagnosis about 35% more were likely to survive another 30 days compared to patients receiving plasma with a low level of antibodies.
However, the study acknowledged that their findings were limited, mainly because it did not have a placebo comparison.
Dr Scott Gottlieb, a former FDA commissioner, said the treatment might help patients, but it “does not seem like a home run.” He agreed that convalescent plasma “certainly” met the standard for emergency use approval “in the setting of a public health emergency.”
“I think this could be beneficial. It could be weakly beneficial,” Gottlieb said on “Squawk Box.” “It does not look like a home team, but at the moment we are looking for singles and doubles. There will actually be no home runs on the horizon until we can get the other therapeutic antibodies on the market and hopefully eventually vaccines and better therapy. . ”
Lawrence Gostin, a professor and faculty director at the O’Neill Institute for National and Global Health Law at Georgetown University, is skeptical about the effectiveness of treatment. He also said he was concerned that the FDA policy had been under pressure to authorize the treatment before data showed whether it was safe and effective. Trump’s announcement on Sunday night came on the eve of the start of the Republican National Convention and 10 weeks before the November 3 presidential election.
On Saturday, Trump accused FDA commissioner Stephen Hahn of enrolling in clinical trials for Covid-19 vaccines as a therapeutic because of political motivations.
“The EUA was awarded without a published peer review study and rolled out with political fanfare,” said Gostin, also the director of the WHO’s Collaborating Center on National and Global Health Law. “I fear that the scientific integrity of FDA could significantly compromise.”
Dr. Jeremy Faust of Brigham and Women’s Hospital of Boston and Harvard Medical School blew up the FDA, tweeting that “science lost today” and “politics won.”
“Breakthroughs come from randomly controlled tears,” he said. “Do not cherry pick subsets of existing datasets and find one promising find under a sea of disappointment.”
–CNBC’s Sil Fire, Kevin Stankiewicz en Emma Newburger contributed to this article.
.