[ad_1]
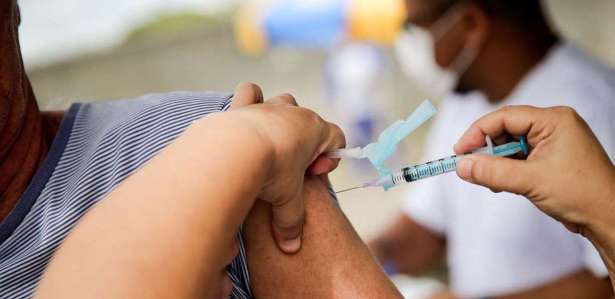
Since the first cases of the new coronavirus were recorded in Wuhan, China, the creation of new vaccines to protect the population has been widely questioned. Latest data from the World Health Organization (WHO) shows that covid-19 has already infected 3,517,345 people and left more than 200,000 dead worldwide. However, to date, there is still no approved covid-19 vaccine for marketing. Only eight are authorized for the test, and another 108 are in the initial study phase.
>> China tests its first coronavirus vaccine in volunteers
>> Coronavirus vaccine should be available in September, Oxford scientists predict
One of the vaccines under test was developed by scientists at the University of Oxford in the United Kingdom. The vaccine, which began testing in April, was called ChAdOx1 nCOV-19 and is made from the weakened genetic material of a common cold virus. The researchers added a genetic material from the coronavirus that is responsible for the production of proteins found on the surface of Sars-COV-2. If efficacy is demonstrated, doses of this vaccine should be available by September 2020.
>> Israel announces discovery of coronavirus antibody
>> The institute seeks to develop an alternative vaccine against the new coronavirus
>> Researchers discover antibodies capable of neutralizing coronavirus
>> WHO Director-General calls for global unity in the fight against coronavirus
>> Brazil has almost 200 volunteers ready to become infected in tests of the coronavirus vaccine
In China, a vaccine began testing on volunteers on April 21, 2020. The researchers will assess whether antibodies have been created and whether the volunteers have become immune to the virus.
In Brazil, researchers at the Butantan Institute in São Paulo are developing a vaccine based on a mechanism used by some bacteria to trick the human immune system. In the study, the researchers will use tiny bubbles, or vesicles, made in the lab that will bind to vesicle proteins on the surface of the new coronavirus. The goal is that, in contact with the defense system, the bubbles create an immune memory in the body and stimulate the production of specific antibodies against covid-19.
Stages of vaccine development.
Although eight vaccines have been launched so far for testing, pediatrician Eduardo Jorge, one of the Vaccine clinic partners, warned about the time to prepare a vaccine. According to him, a vaccine can take ten years to develop.
“We have to remember that it is a new medicine, a new vaccine. It is one thing to have studied 10,000 people. Another thing is to apply this vaccine to millions of people,” he said.
Step 1 – Step 1 consists of testing animals, such as mice. This first step can only be performed after encoding the virus, understanding the immune response, having the perspective of what type of vaccine (attenuated, inactivated virus vaccines, etc.), among other processes included in the so-called preclinical phase.
Step 2 – After testing on animals, the vaccine begins to be tested on people. According to the doctor Eduardo Jorge, in this process between 100 and 300 people are tested. The eight vaccines under study against the new coronavirus are at this stage.
Step 3 – At this stage, between 5,000 and 10,000 people are vaccinated. According to Eduardo Jorge, a vaccine can only be released for marketing after the study in at least this number of people. “To give you an idea, the rotavirus vaccine study was conducted on 80,000 people in various parts of the world before it was released,” he said. After the third phase, the vaccine will be registered and approved.
Step 4 – After the vaccine is released for use and made available to producers, it is necessary to monitor how it will behave in other people. “This is the follow-up phase after the vaccine is already in use in the first few months. The main goals of the phase 4 study are exactly to detect those rare adverse events that were not seen in the phase 3 study,” he explained.
In the pediatrician’s evaluation, the new coronavirus vaccine will be the fastest vaccine to be developed, and that, in five months, scientists have managed to carry out work carried out, generally, in five years. “We hope that these phase 2 studies will finish in June or July, and we will start the phase 3 studies right away. In my opinion, I think it will be the fastest vaccine ever developed in human history. But I have no expectations that that happens before the end of the year. There is a promise from the laboratory for October, but I think it is very exciting. Next year, for sure, we will have some of these vaccines, “he said.
According to him, this advance in stages was only possible because China encoded the virus as early as December, and that it already has the platform for two other coronaviruses, these are: Severe Acute Respiratory Syndrome (SARS), identified in 2003, and the Syndrome Middle East respiratory disease (Mers), discovered in 2012.
The doctor also stressed that until the vaccine is ready, it is necessary to maintain quarantine. “The key to the response to this pandemic is in the vaccine. Until the vaccine arrives, we have to be in social isolation. I only see these two important things for this pandemic. Before the vaccine, do not loosen the isolation,” he stressed.
Subscribe to the new JC newsletter and stay well informed about the coronavirus
Every day, from Sunday to Sunday, always at 8 pm, Jornal do Commercio publishes a new newsletter directly to your email on the most current coronavirus topics in Pernambuco, Brazil and the world. And how do I receive it? It is simple. Interested parties can subscribe to this and other newsletters through the link jc.com.br/newsletter or in the box located at the end of the articles.
What is the coronavirus?
Coronavirus is a family of viruses that cause respiratory infections. The new coronavirus agent was discovered on 12/31/19 after reported cases in China. The first human coronaviruses were first isolated in 1937. However, it was in 1965 that the virus was described as coronaviruses, due to the microscopic profile, which looks like a corona.
Most people become infected with common coronaviruses throughout their lives, and young children are more likely to become infected with the most common type of the virus. The most common coronaviruses that infect humans are the coronavirus alfa 229E and NL63 and the coronavirus beta OC43, HKU1.
How to prevent coronavirus?
The Ministry of Health provides basic care to reduce the overall risk of contracting or transmitting acute respiratory infections, including the coronavirus. Among the measures are:
- Wash your hands frequently with soap and water for at least 20 seconds, respecting the 5 hygiene moments. If soap and water are not available, use an alcohol-based hand sanitizer.
- Avoid touching your eyes, nose, and mouth with your unwashed hands.
- Avoid close contact with sick people.
- Stay home when you are sick.
- Cover your mouth and nose when you cough or sneeze with a disposable tissue and throw it away.
- Clean and disinfect frequently touched objects and surfaces.
- Health professionals should use standard precautions, contact measures and drops (surgical mask, gloves, non-sterile apron and glasses).
To perform procedures that generate aerosolization of respiratory secretions such as intubation, airway aspiration or sputum induction, precautions with aerosols must be taken, with the use of an N95 mask.
See step by step how to wash your hands correctly
[ad_2]