[ad_1]
A panel of external advisers from the US Food and Drug Administration voted overwhelmingly Thursday in favor of backing the emergency use of Pfizer’s coronavirus vaccine, paving the way for the agency to authorize the injection for a nation that has lost more than 285,000 lives to COVID-19.
The FDA is expected to authorize emergency use within days. Distribution and inoculations in the United States are expected to begin almost immediately thereafter.
The committee voted 17 to 4 that the known benefits of the vaccine outweighed the risks of getting the shot for people 16 and older, and 1 panel member abstained.
“This is a historic moment,” said Eric Dickson, CEO of UMass Memorial Health Care, who was not on the advisory panel, after the vote. He called the vaccine from Pfizer and its German partner BioNTech “the best solution to get us out of our current situation and help us save lives.”
Pfizer had asked for the two-dose vaccine to be approved for use in people ages 16 to 85. Several members of the advisory panel discussed whether 16- and 17-year-olds should be included in the recommendation. In the end, they voted on the issue raised by the FDA, which included 16-17 year olds.
It was unclear why four panelists voted against the authorization, but several mentioned that they were uncomfortable with including 16 and 17-year-olds, arguing that the risk to those people was low and the evidence at trial was scant. .
“The final decision on whether to authorize the vaccine for emergency use will be made by career FDA officials,” the agency said in a statement.

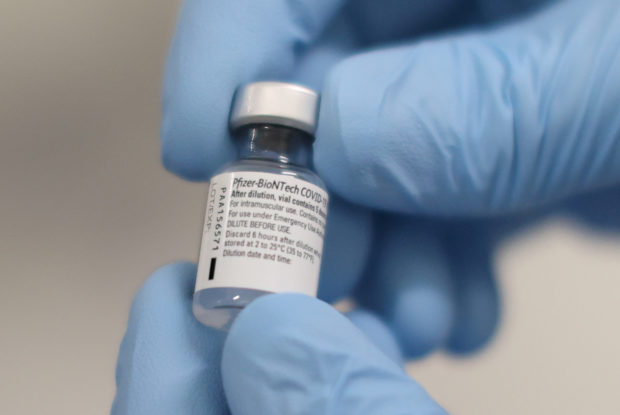
FILE PHOTO: A vial of the Pfizer / BioNTech COVID-19 vaccine is seen before being administered at the Royal Victoria Hospital in Belfast, Northern Ireland, on December 8, 2020. Liam McBurney / Pool via REUTERS
The panel also discussed concerns raised by two reports of severe allergic reactions among vaccine recipients in Britain and what to advise pregnant women, who were excluded from the study. Women of childbearing age make up a large proportion of healthcare workers, who will be among the first to receive the vaccine.
The FDA said during the panel meeting that there was not enough data to support or contradict the use of the vaccine in pregnant women. The agency recommends that you make the decision on your own with the advice of your doctors.
Dr. Gregory Poland, a virologist at the Mayo Clinic in Rochester, Minn., Who has previously served two terms on the FDA’s advisory panel, said he was surprised that the advisers did not issue more warnings about pregnant women.
FDA will review allergy problem
The assessors also spent a large portion of the meeting discussing Pfizer’s plan to give volunteers who received a placebo in its trial the option to receive the vaccine when they are eligible to receive it based on recommendations set forth by state and local health officials. .
Much of that focused on how an emergency authorization would affect the scientific integrity of Pfizer’s ongoing study and how other vaccines will be studied in the future.
Pfizer believes that it should offer the vaccine to people in its trial’s placebo group as they become eligible for the injection.
The concern, voiced by both the FDA and members of the advisory panel, is that telling trial participants who received the placebo and who received the vaccine, which is called “unmasking,” will make it difficult to collect safety data and the efficacy required to obtain full FDA approval of the vaccine.
Documents prepared by the FDA before the meeting did not point to any new safety or efficacy issues, raising optimism that the United States would soon follow the United Kingdom and Canada in licensing the vaccine.
Britain’s health regulator on Wednesday advised some people with a history of anaphylaxis, an overreaction of the body’s immune system related to drugs or food, to avoid receiving the vaccine.
Dr. William Gruber, Pfizer’s senior vice president of clinical vaccine research and development, told the panel that they “saw no serious allergic reactions to the vaccine” among the nearly 44,000 volunteers enrolled in the trial.
However, an FDA official said the agency has asked Pfizer to add serious allergic reactions to its plans to study safety issues related to the vaccine once it is licensed.
Pfizer and BioNTech said last month that a two-dose regimen of the vaccine was 95% effective in preventing COVID-19 disease, and detailed data published in agency documents showed that the vaccine began to show some protection even before the volunteers received a second dose.
The papers also revealed safety data, including cases of Bell’s palsy among volunteers in the placebo and vaccine groups, although they said cases in the trial occurred at the same rate as in the general population. Other reactions included fever, fatigue, and chills.
For more news on the new coronavirus, click here.
What you need to know about the coronavirus.
For more information on COVID-19, call the DOH hotline: (02) 86517800 local 1149/1150.
The Inquirer Foundation supports our leaders in healthcare and still accepts cash donations to be deposited into the Banco de Oro (BDO) checking account # 007960018860 or donate through PayMaya using this link .
Read next
Subscribe to INQUIRER PLUS to get access to The Philippine Daily Inquirer and more than 70 other titles, share up to 5 gadgets, listen to the news, download from 4am and share articles on social media. Call 896 6000.
[ad_2]

