[ad_1]

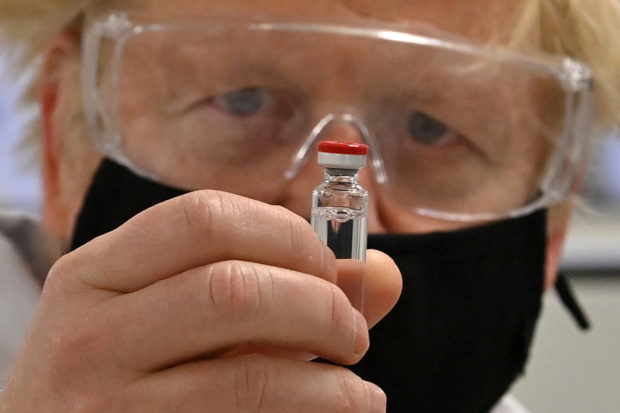
British Prime Minister Boris Johnson poses for a photo with a vial of the University of Oxford / AstraZeneca COVID-19 candidate vaccine, known as AZD1222, at Wockhardt’s pharmaceutical manufacturing facility in Wrexham, Wales, UK, on November 30, 2020. Paul Ellis / Pool via REUTERS / File Photo
LONDON – Britain on Wednesday became the first country to approve the coronavirus vaccine developed by the University of Oxford and AstraZeneca, in the hope that rapid action will help it stop a record rise in infections caused by a highly contagious virus.
The government of Prime Minister Boris Johnson, which ordered 100 million doses, had already accelerated the approval of a vaccine developed by Pfizer and Germany’s BioNTech, and administered hundreds of thousands of injections weeks before the countries of the European Union and the United States. .
Although cheaper and easier to distribute than rival vaccines, the AstraZeneca / Oxford injection has been plagued with questions about its most effective dose since data published last month showed some surprising results.
While other regulators have taken a more cautious approach, Britain’s MHRA went to great lengths to say it had resolved initial concerns and unexpectedly found an 80% success rate for the administration of two full doses, with three months apart, higher than the average that the developers themselves had found.
The government plans to take advantage of by administering the first dose to a greater number of people at increased risk of COVID-19 before starting to administer the boosters.
An advisory body recommended doing the same with the Pfizer injection, although Pfizer said its vaccine had not been tested in different dosing schedules.
Jeremy Farrar, one of Britain’s leading public health experts, said the approval of the AstraZeneca / Oxford injection was to be celebrated, but urged continued scrutiny and recommended that developers run a randomized trial to test the timing of the second dose.
Jonathan Stoye, a virologist at the Francis Crick Institute, agreed that questions remain about the vaccine’s true efficacy, how well it works in older people, and what data exists to support the change in dosing interval.
“In light of the increasing number of cases, the approval … is great news,” he said. “However, the reported news leaves a number of important questions unanswered, particularly regarding the long term.”
The government said it would not recommend one vaccine over another for different population cohorts, although data on the efficacy of the AstraZeneca / Oxford injection in older people is currently limited.
Infections soar
Britain has already recorded more than 70,000 deaths from COVID-19. On Tuesday it reported a record one-day increase of 53,135 new coronavirus infections, and fears that hospitals will soon be overloaded in their peak winter months.
The AstraZeneca / Oxford vaccine could also be a game changer for global immunization. Countries with relatively basic healthcare infrastructure have high hopes for an injection that, unlike Pfizer’s, can be stored and transported in normal refrigeration, rather than supercooled to -70 degrees Celsius (-94 Fahrenheit).
The Serum Institute of India (SII) in India, the world’s largest vaccine producer, has manufactured around 50 million doses of Oxford vaccine.
Experts from India’s drug regulator met on Wednesday to consider an emergency approval and will discuss the issue again on Friday. Chile is also interested.
World Health Organization spokesman Tarik Jasarevic said the latest vaccine was important for its “attributes of delivery, potential scale and affordability.”
Helen Fletcher, a professor of immunology at the London School of Hygiene and Tropical Medicine, hailed a “tipping point” for the pandemic, which has already killed 1.7 million people worldwide, wreaking havoc on the world economy and disrupted the normal lives of billions.
“With more than 30 supply agreements and partner networks established globally, the Oxford / AstraZeneca vaccine could curb the pandemic and should save many lives over the next year,” he said.
EU DECISION SOON?
The EU regulator says it has not yet received full data on the AstraZeneca injection and is unlikely to be able to approve it next month, although Germany’s top vaccines official said an ongoing review of the data meant it should be possible. a quick decision.
Canada said it needs more information from the British drugmaker as part of its ongoing review.
UK COVID-19 vaccine chairman Wei Shen Lim said a single dose of the AstraZeneca / Oxford vaccine was around 70% effective from 21 days until a second dose was administered at 12 weeks.
The UK Medicines and Healthcare Products Regulatory Agency (MHRA) cleared up a question raised by the Oxford data, saying that a 90% success rate for a half dose followed by a full dose had not stood up to analysis.
A Reuters investigation detailed how the apparently most effective dosage regimen was the result of a miscalculation.
However, Munir Pirmohamed, chairman of a government working group on COVID-19 vaccines and involved in the approval, said that when two full doses were administered three months apart, “the effectiveness was high, up to 80% … Which is the reason for our recommendation. “
AstraZeneca Chief Executive Pascal Soriot told BBC radio that the UK should be able to vaccinate tens of millions of people by the end of the first quarter. The firm said it hoped the vaccine would work against the new variant.
Health Secretary Matt Hancock told Sky News that he was “very confident that we can vaccinate enough vulnerable people before spring so that we can now see our route out of this pandemic.”
gsg
For more news on the new coronavirus, click here.
What you need to know about the coronavirus.
For more information on COVID-19, call the DOH hotline: (02) 86517800 local 1149/1150.
The Inquirer Foundation supports our leaders in healthcare and still accepts cash donations to be deposited into the Banco de Oro (BDO) checking account # 007960018860 or donate through PayMaya using this link .
Read next
Subscribe to INQUIRER PLUS to get access to The Philippine Daily Inquirer and more than 70 other titles, share up to 5 gadgets, listen to the news, download from 4am and share articles on social media. Call 896 6000.
[ad_2]

