[ad_1]
The manufacturing delays mean only 4 million doses of the candidate vaccine developed by pharmaceutical company AstraZeneca AZN,
and the University of Oxford will be delivered to the UK, below the 30 million doses the country expected to receive before the end of the year.
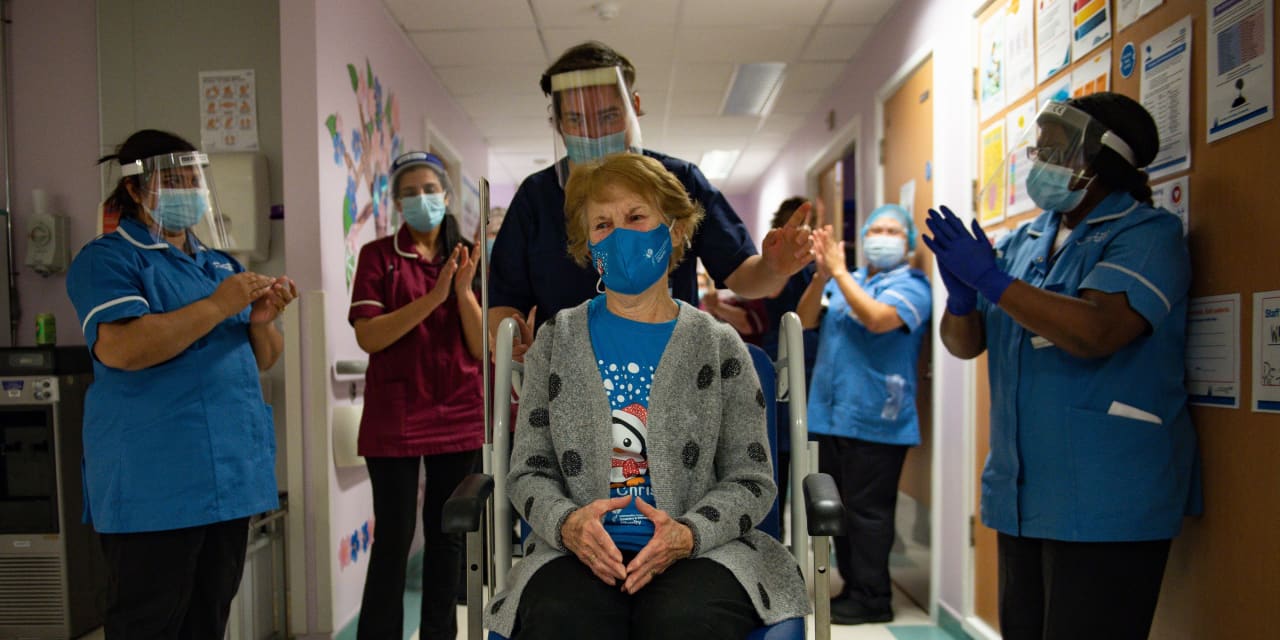
News of the shortfall comes as the UK became the first country to embark on a mass immunization program, and National Health Service hospitals on Tuesday began administering 800,000 injections of the vaccine developed by US drugmaker Pfizer PFE. .
and its German partner BioNTech BNTX,
UK regulators granted emergency clearance for the vaccine on December 2. European Union regulators are also reviewing the injection, along with rival vaccines being developed by American biotech company Moderna MRNA.
and the AstraZeneca AZN,
candidate.
The Pfizer and Moderna vaccines have been shown to be 95% effective in protecting people against coronavirus.
On Tuesday, Margaret Keenan, who will turn 91 next week, was the first person to be hit by Nurse May Parsons at 6.31am GMT at Coventry University Hospital in central England, in what the health secretary Matt Hancock called it ‘V Day’. ‘
“I feel very privileged to be the first person to be vaccinated against COVID-19, it is the best anticipated birthday gift I could wish for because it means that I can finally look forward to spending time with my family and friends in the new year after being on my own during most of the year, ”Keenan told the BBC.
Read: Who will get priority for the Pfizer-BioNTech COVID-19 vaccine?
The NHS said the vaccine is offered in some hospitals to some people over the age of 80, people who work in nursing homes and healthcare workers at high risk. “The vaccine will be offered more widely, and elsewhere, as soon as possible,” the NHS said in a statement.
Prime Minister Boris Johnson tweeted: “The UK’s first COVID-19 vaccines begin today. “Thank you to our NHS, to all the scientists who worked so hard to develop this vaccine, to all the volunteers, and to all who have followed the rules to protect others. Let’s get through this together. “
The UK has secured 800,000 doses of the Pfizer-BioNTech vaccine, to be delivered in the coming weeks. He has placed orders for 40 million in total, enough for 20 million people, since two injections are required at least 21 days apart.
The wide distribution of the Pfizer vaccine has been hampered by logistical problems, as the injection must be stored at minus -70 degrees Celsius (-94 degrees Fahrenheit). In comparison, the AstraZeneca candidate vaccine can be stored at normal temperatures similar to that of a refrigerator, making it easy to store and transport.
Read: Boris Johnson warns of ‘immense logistical challenge’ as UK becomes first to authorize use of Pfizer-BioNTech COVID-19 vaccine
The immunization program will be a “marathon, not a sprint,” said Professor Stephen Powis, NHS England’s national medical director, adding that “this really feels like the beginning of the end.”
On Tuesday, Hancock told the BBC he had “high hopes” that UK regulators would approve the AstraZeneca vaccine in the coming weeks. Full data from the late-stage clinical trials, involving 24,000 people, have yet to be published.
A US study of the AstraZeneca vaccine involving about 30,000 volunteers is currently underway and should release data by the end of January.
Read: What side effects, if any, can you expect from the COVID-19 vaccine injection?
However, the UK government’s vaccine task force said on Monday that only 4 million doses of the AstraZeneca vaccine, imported from the Netherlands and Germany, would be delivered this year, compared to the 30 million injections it expected. , after delays in manufacturing.
“Accelerating vaccine production and supply involves collaboration with more than 20 supplier partners in more than 15 countries, supported by more than 20 analytical testing sites,” AstraZeneca said in a Nov. 25 statement.
The government is expected to launch a trial in January to examine whether the combination and combination of vaccines provides better protection than two doses of the same, said Kate Bingham, outgoing chair of the UK vaccine task force.
Volunteers participating in the trial will receive an injection of the AstraZeneca vaccine, if approved, and one from Pfizer. Moderna will also be included in the test if her shot is approved, The Guardian reported.
Giving a progress report on the task force’s first six months on Monday, Bingham said the “mix and match” trials weren’t about limited supplies of the vaccine, but about triggering the immune response and durability.