[ad_1]

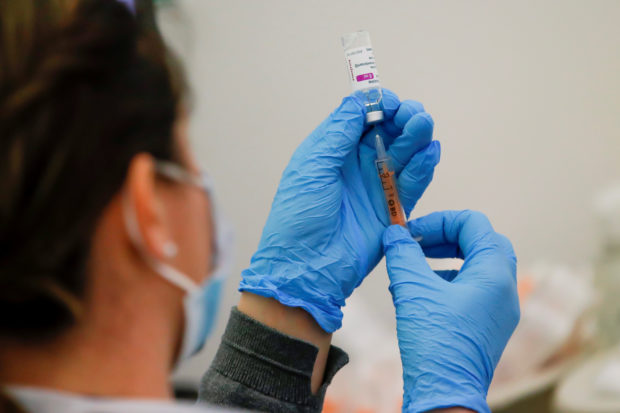
FILE PHOTO: A dose of AstraZeneca COVID-19 vaccine is prepared at a vaccination center at Newmarket Racecourse, amid the coronavirus disease outbreak in Newmarket, Britain, on March 26, 2021. REUTERS / Andrew Couldridge / File Photo
British regulators said Thursday they have identified 30 cases of rare blood clot events after the use of the AstraZeneca COVID-19 vaccine, 25 more than the agency previously reported.
The Medicines and Health Products Regulatory Agency (MHRA) said it had not received such reports of clotting events following the use of the vaccine manufactured by BioNTech SE and Pfizer Inc.
On Friday, the drug regulator told the Financial Times and The Guardian that seven recipients of the AstraZeneca vaccine died after recording the rare blood clotting events. Reuters could not immediately confirm the MHRA figure after business hours.
The MHRA, the European Medicines Agency and the World Health Organization have reiterated that the benefits of the vaccine in preventing COVID-19 far outweigh any possible risk of blood clots.
Some countries are restricting the use of the AstraZeneca vaccine, while others have resumed inoculations as investigations into reports of rare and sometimes serious blood clots continue.
On March 18, the UK drug regulator said there were five cases of a rare cerebral blood clot among the 11 million injections given.
On Thursday, he calculated the count on 22 reports of cerebral venous sinus thrombosis, an extremely rare brain clotting disease, and 8 reports of other clotting events associated with low platelet levels out of a total of 18.1 million doses administered. .
For more news on the new coronavirus, click here.
What you need to know about the coronavirus.
For more information on COVID-19, call the DOH hotline: (02) 86517800 local 1149/1150.
The Inquirer Foundation supports our leaders in healthcare and still accepts cash donations to be deposited into the Banco de Oro (BDO) checking account # 007960018860 or donate through PayMaya using this Link .
Read next
Subscribe to INQUIRER PLUS to get access to The Philippine Daily Inquirer and more than 70 other titles, share up to 5 gadgets, listen to the news, download from 4am and share articles on social media. Call 896 6000.
For comments, complaints or inquiries, please contact us.
[ad_2]

