[ad_1]
China’s approval of its first COVID-19 vaccine for public use on December 30 was good news to usher in the New Year. But some questions have been raised both inside and outside of China, and these are some of the most common:
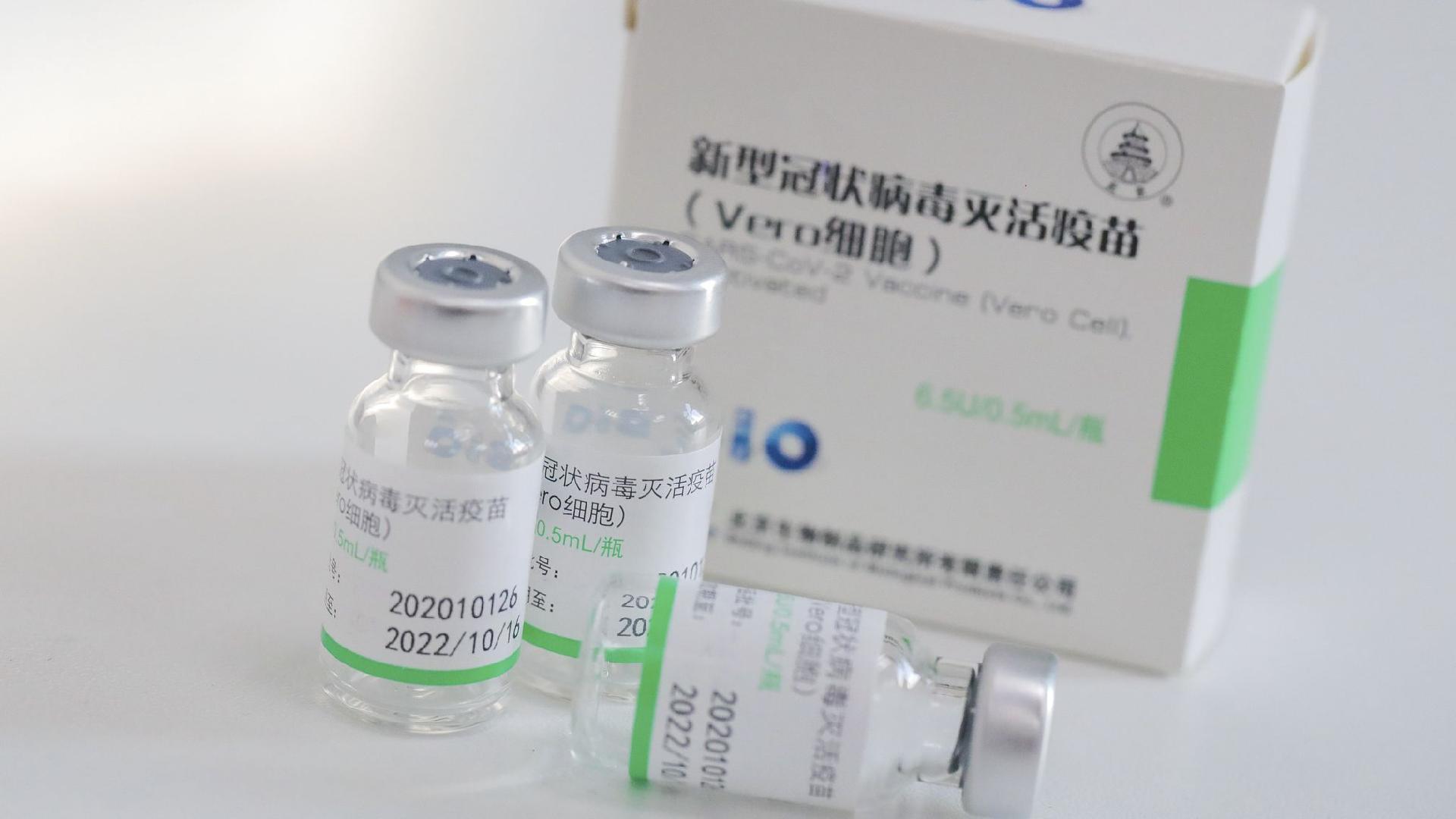
1. Where does the vaccine come from?
The COVID-19 vaccine was developed by a subsidiary of the pharmaceutical giant Sinopharm and obtained a conditional market authorization from the China National Medical Products Administration (NMPA). It is China’s first COVID-19 vaccine approved for general public use.
2. What does conditional authorization mean?
A conditional authorization is used when there is no effective treatment available for a life-threatening disease or when there are incomplete clinical data or registration details. This is not unusual. The HPV vaccine in China also received conditional authorization, which has been in place in China for just over two years.
Vaccines have yet to have certain clinical research results and check many boxes to receive conditional approval for public use. They are also closely monitored and followed up with clinical trial data and research published before they are fully licensed.
3. What is the advantage of this vaccine over existing ones?
Sinopharm’s vaccine is inactive, which means it does not require extreme freezing, making it easy to store and distribute in rural areas and developing countries.
The current vaccines from Moderna and Pfizer-BioNTech are mRNA vaccines, which should be kept at minus 20 degrees Celsius and minus 70 degrees Celsius respectively.
4. Is this vaccine safe?
Based on current available data, the vaccine is safe.
China approved the emergency use of Sinopharm vaccines in July, and by the end of November 2020, more than 450 million doses of vaccines had been administered with no serious side effects reported, according to Zeng Yixin, deputy director of the National Commission. of Health, in a press conference on December 31.
More than 60,000 people of 125 nationalities in many countries have been injected into phase 3 clinical trials, according to Wu Yongling, president of the China National Biotec Group, affiliated with Sinopharm.
5. What do efficacy and effectiveness mean?
The approved inactivated vaccine has been shown to be 79.34 percent effective against COVID-19, based on interim results of phase 3 clinical trials. It means that people injected with the vaccine are 80 percent protected against infection, compared to those who are completely vulnerable without the vaccine.
Efficacy, on the other hand, takes into account real-world circumstances, such as how quickly the virus spreads, the different responses of individuals’ immune systems, and whether people have previously been exposed to the virus. The effectiveness is generally less than the effectiveness.
6. Am I definitively protected by the vaccine after inoculation?
No, a good sense of protection is still needed.
Interim results from phase 3 clinical trials of the vaccine show an efficacy of 79.34 percent, but it is not foolproof.
Even after inoculation, people should continue to wear masks in crowded places, wash their hands regularly, and cover a sneeze with their elbow, among other things.
7. Why is it free to the public? Who pays
The National Health Commission announced that the recently approved COVID-19 vaccine will be free to the Chinese public at the December 31 press conference. Specific policies are expected to be implemented in the coming months.
According to the vaccine regulations in China, when a vaccine is included in the Expanded Program on Immunization, the government will cover the cost. If it is a vaccine that is not from the EPI, the beneficiaries of the vaccine must pay.
8. Can everyone get the vaccine?
The vaccine will prioritize high-risk groups, including cold chain workers, border inspectors, doctors, the elderly, people with pre-existing diseases, as well as government employees, community workers, and people with plans. of traveling abroad.
Vaccination will be expanded to the general population as more specific plans are implemented.
But the vaccine may not be for everyone. Those who show severe allergic symptoms to the first shot of the vaccine should avoid the second.