[ad_1]
MANILA, Philippines – The Armed Forces of the Philippines said they would launch their own investigation into the clandestine inoculation of President Rodrigo Duterte’s close security team with a vaccine for COVID-19 not yet authorized by local regulators.
The inoculation had sparked accusations that the Presidential Security Group (PSG) and Malacañang may have violated the law in their desire to protect Duterte from SARS-CoV-2, the virus that causes COVID-19.
The secret vaccination was criticized by the public for undermining the most vulnerable sectors, in particular healthcare workers, who should have been the first to be vaccinated during the pandemic.
In a statement Thursday, Major General Edgard Arevalo, an AFP spokesman, said that the chief of staff, General Gilbert Gapay, ordered a “thorough investigation into the factual circumstances surrounding the incident.”
However, the AFP supported the statement of the PSG commander, Brig. Jesús During III justifies the inoculation of Duterte’s bodyguards “to greatly reduce the possibility of being a source and carrier of this virus and consequently infecting the President, whose good health and well-being are the main concern of PSG,” said Arevalo.
He said that Gapay “was not a party to nor was he aware of the circumstances related to obtaining these vaccines, their source and the administration of them to the PSG soldiers.”
During had said that PSG members vaccinated each other in September and October, but declined to identify the donor or manufacturer of the “donated” vaccine.
Separate investigations
The Food and Drug Administration (FDA), Customs Office and the National Bureau of Investigation are conducting separate investigations into how the unregistered vaccine made its way into the country and was administered to presidential guards.
The Senate will meet as a plenary committee to open its own investigation. The senators will summon During to clarify how the vaccine doses were obtained.
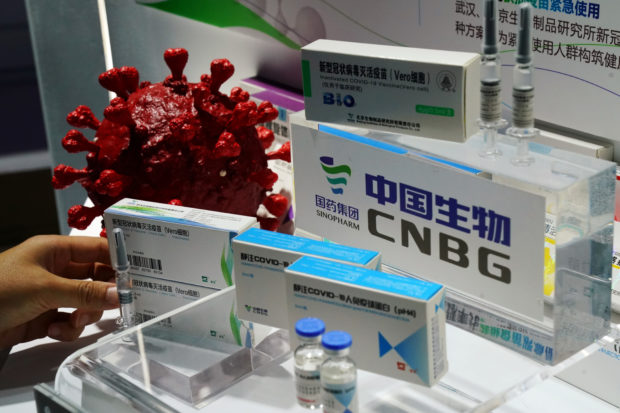
Government officials finally admitted that the vaccine for COVID-19 was developed by the Chinese pharmaceutical company Sinopharm.
The Sinopharm vaccine has not yet been approved by the FDA for use in the country.
During said he would assume “full responsibility” for vaccinating PSG members with an unauthorized vaccine.
Critics and some legislators said PSG violated Republic Law No. 9711, or the FDA Law of 2009, which prohibits the manufacture, import, export, sale, distribution, transfer, and non-consumer use of products. not registered. Violators can face jail terms from one year to 10 years.
Defense Secretary Delfin Lorenzana himself said that the Sinopharm vaccine was “smuggled” into the country.
According to the government’s vaccination priority list, the military ranked fifth among the priority recipients, behind front-line health workers, select government office workers, the elderly and the homeless.
Distributor denies participation
In a radio interview on Friday, two representatives of a company claiming to be the sole distributor of the Sinopharm vaccine in the country denied their involvement in the inoculation of PSG members.
The two men told DZBB radio that their Makati City-based company, MKG Universal Drugs Trading Corp., would follow the law when distributing the Sinopharm vaccine. They refused to reveal their identities for security reasons.
They said they had obtained an application from the FDA and submitted it to Sinopharm in China, which would then apply for an emergency use authorization (US) from the FDA.
The FDA said that so far only US-based Pfizer / BioNTech has applied for the US for its COVID-19 vaccine.
A USA is a mechanism intended to accelerate the availability of a drug or vaccine to the public in times of a health emergency, such as the current pandemic.
Even if the vaccine development process is still ongoing, an EUA may be issued if “convincing” safety and efficacy data are available.
This authorization, however, does not exempt the manufacturer from completing the development of its product and requesting prequalification once it is licensed.
The Chinese government just approved the use of Sinopharm’s COVID-19 vaccine for general use in its population on Thursday.
This came after it announced that the vaccine it developed had a 79 percent efficacy rate based on an analysis of its phase 3 clinical trials.
Sinopharm had not published data from its clinical trials, saying they would be published “later” in Chinese and foreign medical journals.
For more news on the new coronavirus, click here.
What you need to know about the coronavirus.
For more information on COVID-19, call the DOH hotline: (02) 86517800 local 1149/1150.
The Inquirer Foundation supports our leaders in healthcare and still accepts cash donations to be deposited into the Banco de Oro (BDO) checking account # 007960018860 or donate through PayMaya using this link .
Read next
Subscribe to INQUIRER PLUS to get access to The Philippine Daily Inquirer and more than 70 other titles, share up to 5 gadgets, listen to the news, download from 4am and share articles on social media. Call 896 6000.
[ad_2]

