
[ad_1]
Pfizer and BioNTech have surprised the world, and given it hope, with the preliminary results of the phase 3 clinical trial of their coronavirus vaccine.
They announced on Nov. 9 that initial analysis of data from the phase 3 clinical trial, which is still ongoing, showed the vaccine was 90 percent effective. The unexpectedly high figure still needs to be confirmed in greater numbers and over time. The vaccine is simple to manufacture, but its storage is more complex (the vaccine must be kept at very low temperatures). Large-scale production is expected to begin shortly.
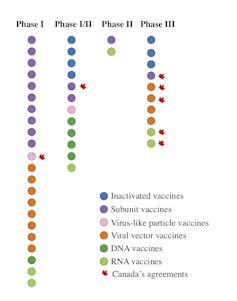
Labs around the world are in a race to produce a COVID-19 vaccine. While the finish line is now in sight for Pfizer and BioNTech, the World Health Organization (WHO) lists 202 candidate vaccines, 47 of which are in human trials.
Canada hasn’t put all of its eggs in one basket in its plan to protect Canadians from the new coronavirus. In addition to Pfizer and BioNTech, it has signed six other contracts: Moderna, Sanofi and GlaxoSmithKline, Johnson & Johnson, Novavax, AstraZeneca and Medicago. Canada recently announced that it had reserved an additional 56 million doses of vaccines from Pfizer and BioNTech, in addition to the 20 million doses it had already purchased, bringing its order to 76 million.
(World Health Organization)
Canada has ensured access to a total of 414 million doses of COVID-19 vaccines from different sources. More importantly, Canada has made sure that it has diversified the types of vaccines it will have.
Scientists are using different platforms to develop COVID-19 vaccines. Some candidate vaccines in clinical trials exploit mechanisms already used in other vaccines. Others are based on innovative approaches that have never been tried before. Here is an overview of the different types of vaccines.
Images created with Mol * (Almo and others (2020) PBD ID 6X6P. Figure created with BioRender.com
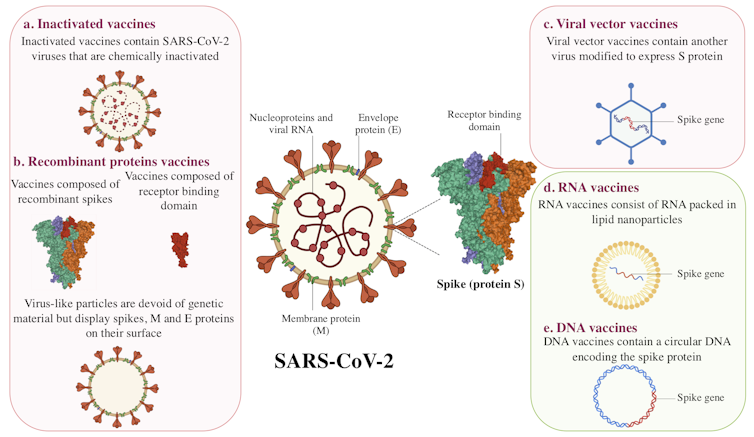
Inactivated vaccines
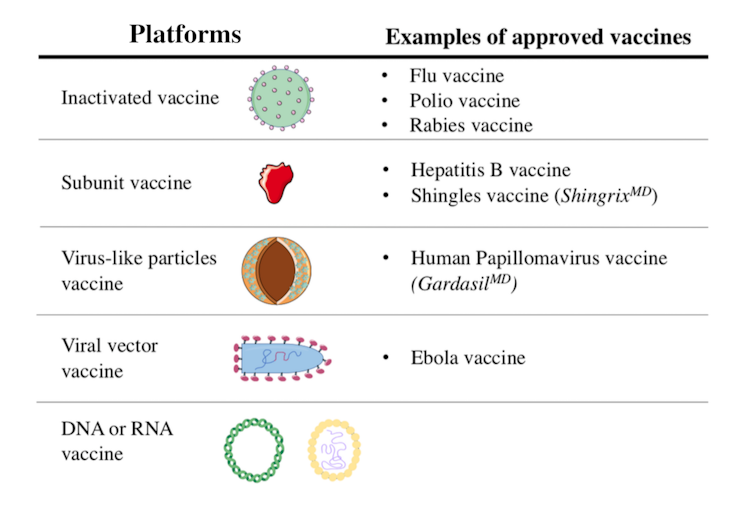
Inactivated vaccines have been used since the 1880s. The viruses in these vaccines have been rendered inactive by chemical treatment, as with the SARS-CoV-2 candidate vaccines, or by physical treatment.
With this type of vaccine, the immune system encounters the virus in its entirety. It can mount a defense when it detects viral spike protein (also called spike or protein S), envelope, and nucleoprotein.
Currently, seven inactivated candidate vaccines are being tested in humans. Of these, three are in phase 3 clinical trials. Unlike Phase 1 and Phase 2, which are used to evaluate the tolerability, safety, and ability of a vaccine to induce an immune response, a Phase 3 clinical trial allows for scientists to test its effectiveness.
Recombinant proteins
Recombinant protein vaccines are divided into two categories: subunits and virus-like particle vaccines.
For subunit protein vaccines, a viral protein is produced in large quantities in a living “factory,” such as a bacterium, a plant, a mammalian cell, or an insect. When the viral protein is presented to the immune system, it triggers a reaction.
The 13 candidate vaccine subunits currently in phase 1, 2 or 3 clinical trials are comprised of the entire spike protein or a specific portion of the spike protein called the “receptor-binding domain.”
Virus-like particle vaccines are made up of a set of viral proteins that mimic the shape of the virus. This “pseudovirus” particle is an empty shell, devoid of genetic material and non-infectious, but this does not prevent the immune system from recognizing it.
Viral vector vaccines
This approach is based on the use of a non-pathogenic or low-human-hazard virus. In the case of 12 such candidate vaccines currently being studied in humans, the viral vectors are mostly adenoviruses. They represent a large group of viruses that can cause colds and conjunctivitis, among other symptoms.
Used as Trojan horses, these viruses are modified to express the SARS-CoV-2 spike protein after vaccination. Viral vector vaccines are a recent strategy, but they were used in the development of the Ebola virus vaccine.
RNA and DNA vaccines
Despite differences in composition, DNA and mRNA (messenger RNA) contain genetic information for protein production. While an RNA molecule can directly produce that information, DNA requires an intermediate transcription step.
RNA or DNA vaccine candidates differ from other strategies in two ways. First, it is a novel strategy: there are no RNA or DNA vaccines on the market. Second, they are the only candidates for vaccines composed solely of genetic material.
In the case of RNA vaccines, the messenger RNA molecules are encased in lipid nanoparticles. Once the vaccine is injected, the RNA serves as a template for the cells of the body to produce a viral protein, the spike protein, to be precise.
DNA vaccines, on the other hand, are made up of circular DNA (called a plasmid). This DNA will be transcribed into RNA, which will again serve as a template.
Six RNA vaccine candidates are currently being tested in humans, two of which are in Phase 3. All five DNA vaccine candidates are in Phase 1 and 2 clinical trials.
Canada in the vaccine race
The following is an overview of each company that has an agreement with the federal government, the type of vaccine in development, and the number of doses reserved by Canada.
Pfizer / BioNTech: 76 million reserved doses
BioNTech, in Germany, and Pfizer, in the United States, are jointly developing an RNA vaccine. This candidate codes for the manufacture of the peak protein.
The phase 3 clinical study continues with more than 43,000 patients in the United States, Argentina, Brazil, Germany, Turkey, and South Africa. The vaccine is reported to be over 90% effective and has not caused serious side effects.

(AP Photo / Virginia Mayo)
Despite these encouraging preliminary results, Pfizer and BioNTech have not yet crossed the finish line. Detailed data have not been published and questions remain, including the age and risk factors of vaccinated people and the duration of protection. The clinical trial is ongoing and more data will be analyzed.
Modern: 56 million reserved doses
The candidate vaccine from Moderna, a US-based biotech company, is an RNA vaccine. Once injected, it allows expression of the spike protein. Currently, in phase 3 clinical trials, the vaccine is being tested in 30,000 people in different regions of the United States.
Johnson and Johnson: 38 million reserved doses
Johnson & Johnson’s candidate is a viral vector vaccine composed of a human adenovirus that has been modified to render it incapable of multiplying, but capable of expressing the SARS-CoV-2 spike protein. The phase 3 clinical trial, which began in September 2020, is underway in several countries and will have 60,000 participants.
AstraZeneca / University of Oxford: 20 million reserved doses
The University of Oxford has partnered with AstraZeneca on a viral vector vaccine. The candidate vaccine is composed of a modified chimpanzee adenovirus that expresses the peak protein. It is in phase 3 clinical trials.
Novavax: 76 million reserved doses
The vaccine candidate from Novavax, a US company, is based on the recombinant protein strategy. It is made up of spike protein and an adjuvant, a booster used in vaccines to boost the immune response. The phase 3 clinical trial started in September 2020 and involves 10,000 participants in the UK.
Created from Servier’s medical art (http://servier.com/Powerpoint-image-bank). Servier Medical Art from Servier is licensed under a Creative Commons Attribution 3.0-Unported license.
Sanofi / GSK: 72 million reserved doses
The candidate of the French company Sanofi and the British giant GlaxoSmithKline (GSK) is composed of an adjuvant and recombinant version of the peak protein, produced in a living factory (baculovirus). Phase 1 and Phase 2 clinical trials are currently testing its safety, tolerability, and ability to induce an immune response.
Medicago: 76 million reserved doses
Developed by the Quebec-based company Medicago, this candidate vaccine is made up of virus-like particles. These are produced in plants infected with bacteria that have been genetically modified to produce various SARS-CoV-2 proteins. These plants thus become production plants.
Researchers can extract the particles from the leaves and purify them. The Medicago candidate vaccine is currently in phase 1 clinical trials and the results are also promising.