[ad_1]
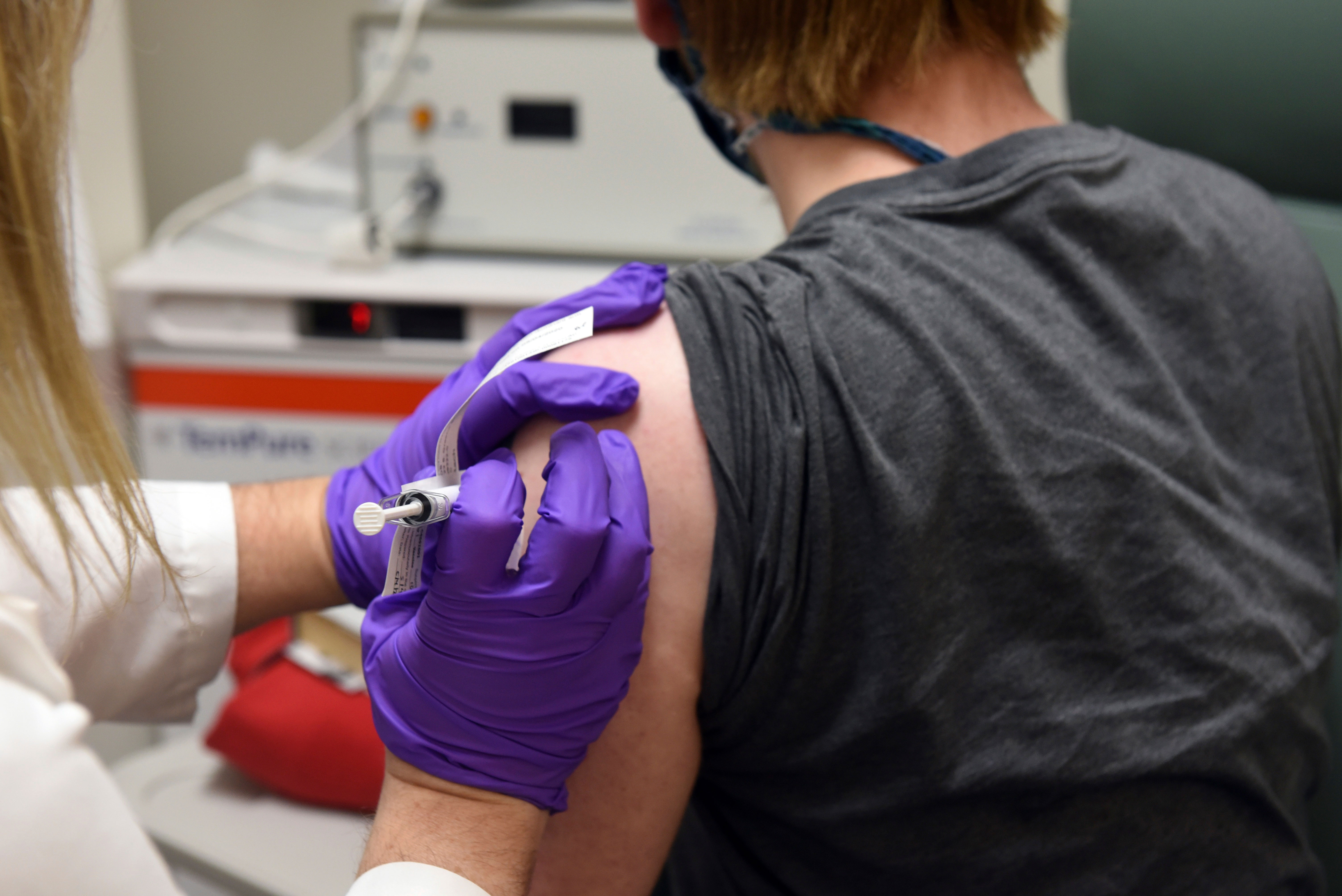
The U.S. Food and Drug Administration may not complete its review of Pfizer’s Covid-19 vaccine until Christmas, according to the head of the federal network that coordinates clinical trials of the Covid-19 vaccine.
Pfizer announced Monday that early data shows its vaccine is more than 90% effective.
The pharmaceutical giant says it could apply to the FDA for an emergency use authorization shortly after the vaccine’s safety data is collected next week.
Dr. Larry Corey, who heads the Covid-19 Prevention Network, which is funded by the National Institutes of Health, said he thought it would take the FDA several weeks to finish reviewing the vaccine’s safety and efficacy data, as well as information. about its manufacture.
“I want to set the expectation that this is a great decision,” Corey said.
Alex Azar, the secretary of Health and Human Services, has said that the process to get clearance from the FDA will take several weeks, while Dr. Anthony Fauci says vaccines will likely start a little before Christmas.
Corey noted that it took the FDA more than four weeks to consider a request from Eli Lilly and Co for its antibody therapy. Lilly applied to the FDA for an emergency use authorization on October 7 and received it on November 9.
“You can see that moment as a marker,” Corey said. “There is a lot of data to review.”
Corey believes it will take the FDA about 10 days to review the data from Pfizer’s clinical trials. The agency should also review Pfizer’s manufacturing data.
“I’m not sure how long that review will take, but it could be two weeks. I think it’s reasonable, ”Corey said.
If approved, Pfizer’s vaccine would first be administered to priority groups such as healthcare workers, the elderly, and those with underlying health problems that make them more vulnerable to complications from Covid-19.
Fauci told CNN on Tuesday that the United States “will probably be able to begin distributing vaccines in December.”
He also told Australia’s ABC network that “vaccines will start in December, most likely a little before the Christmas holidays.”
Fauci is director of the National Institute of Allergy and Infectious Diseases, which created the Covid-19 Prevention Network last summer.
Azar said Monday that the process for obtaining authorization for the vaccine from Pfizer would be “within several weeks.”