[ad_1]
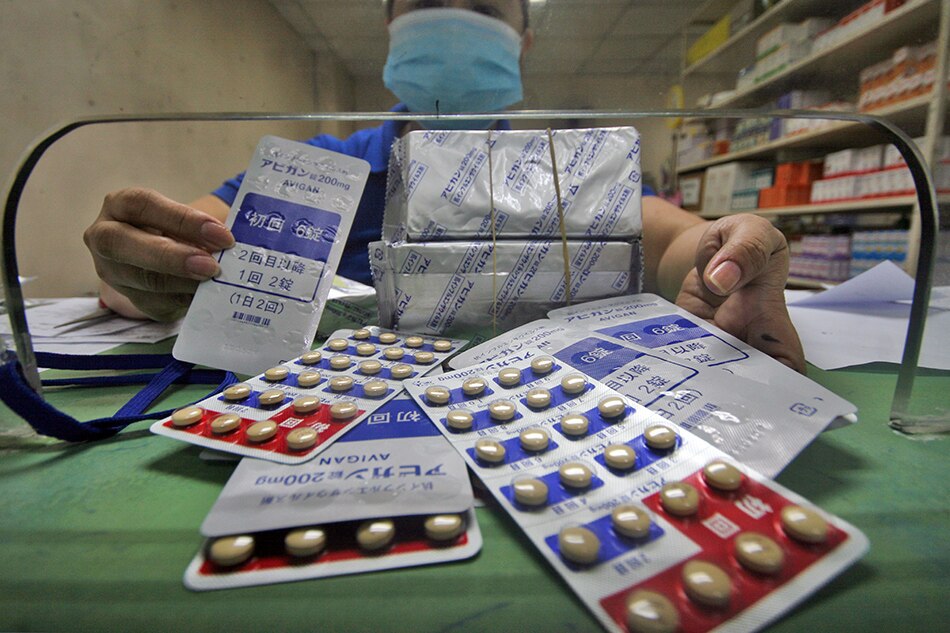
MANILA – The Philippines will finally begin enrolling patients for its clinical trial that aims to test whether the Avigan flu drug is also effective against COVID-19.
The Undersecretary of Health, María Rosario Vergeire, said on Friday that they are waiting for the insurance documents to be finalized.
“Can a minor comment on the legal service for this insurance document (Our legal service has a minor comment for this insurance document),” he said during a virtual briefing, referring to the insurance coverage of the participants
“But we already gave the signal to our proponent, now they are going to start recruiting participants for the trial,” he added.
Vergeire said that once participants are recruited, the trial can officially begin “hopefully next week.”
The trial of Avigan or favipiravir was supposed to start in August, but unsigned documents and other requirements delayed it.
As early as March, the Philippines asked Japan for access to the drug. Received the tablets in August.
Last month, Fujifilm, the company that developed the drug, said its clinical study showed reduced recovery times for COVID-19 patients.
The trial is supposed to last 9 months and will be carried out in 4 hospitals: Philippine General Hospital, Sta. Ana Hospital, Dr. José N. Rodríguez Memorial Hospital and Quirino Memorial Medical Center.
COVID-19, coronavirus, DOH, Department of Health, COVID-19 clinical trials, clinical trials, avigan, favipiravir
[ad_2]