In older adults (65-85 years), the vaccine candidate showed a neutralizing GMT 1.6 times the GMT of the same panel, demonstrating strong immunogenicity in younger and older adults. Across all populations, BNT162b2 administration was well tolerated with mild to moderate fever in less than 20% of participants.
“The entirety of the clinical and preclinical data informed Pfizer and BioNTech’s decision to select BNT162b2 as the main candidate to proceed in pivotal trials,” said Kathrin U. Jansen, Pfizer’s head of fax research and development. “We are particularly pleased to present this early data showing the promising safety and immunogenicity profile of our vaccine candidate from the US trial and we look forward to sharing data from the German trial’s T immune response in the US trial. whole future. “
The data results required the selection of the BNT162b2 candidate for the pivotal Phase 2/3 global study that began in July 2020. Currently, the study enrolled more than 11,000 participants. It is now actively enrolled in the US, Argentina and Brazil. Additional registration is planned in Germany, Turkey and South Africa. The study is a pilot-driven trial designed to enroll up to 30,000 participants between the ages of 18 and 85.
Pfizer and BioNTech added that once the regulatory approval is obtained, they are currently planning to deliver up to 10021 up to 100 million vaccine doses worldwide by 2021 and about 1.3 billion doses.
The BNT162 program is based on BioNTech’s proprietary mRNA technology and backed by Pfizer’s global fax development and manufacturing capabilities. The vaccine development program evaluates at least four experimental vaccine candidates, each representing a unique combination of messenger RNA (mRNA) format and target antigen.
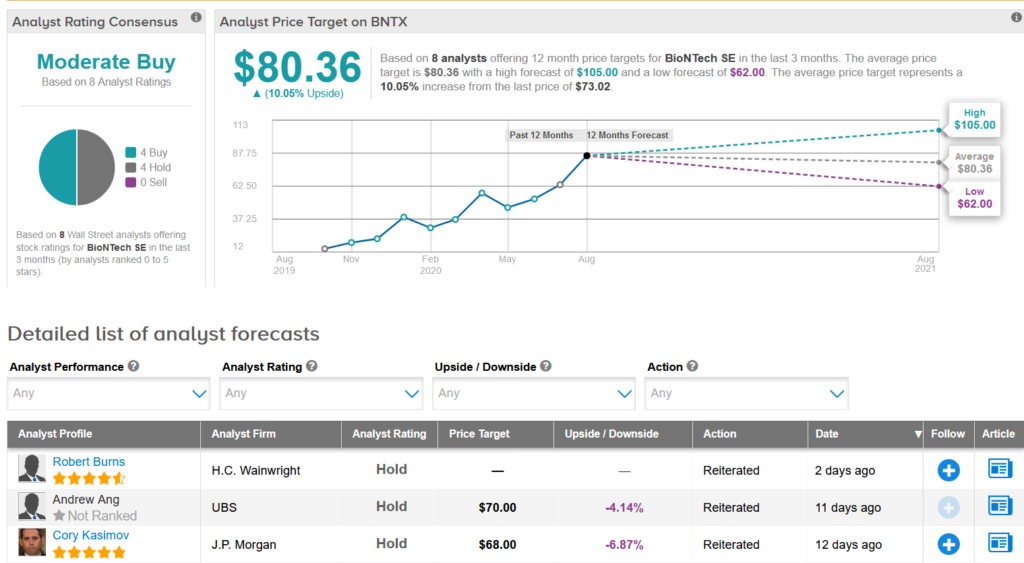
Shares in BioNTech have piked 116% so far this year and analysts predict another potential of 10% to set the average price target at $ 80.36.
Commenting on the trial data, HC Wainwright analyst Robert Burns said BNT162b2 appears to have an improved safety profile, but he nevertheless reiterated a Hold rating on the front.
“Despite the encouraging data, we can not predict with certainty what the vaccine will achieve in sales, given the complexity and the constantly evolving epidemiology of COVID-19 and the rapidly changing nature of the competitive landscape,” Burns wrote in a note to investors.
Overall, Wall Street analysts have a cautiously optimistic outlook on the stock with a consensus from Moderate Buy. (See BioNTech stock analysis on TipRanks).

More recent articles from Smarter Analyst: