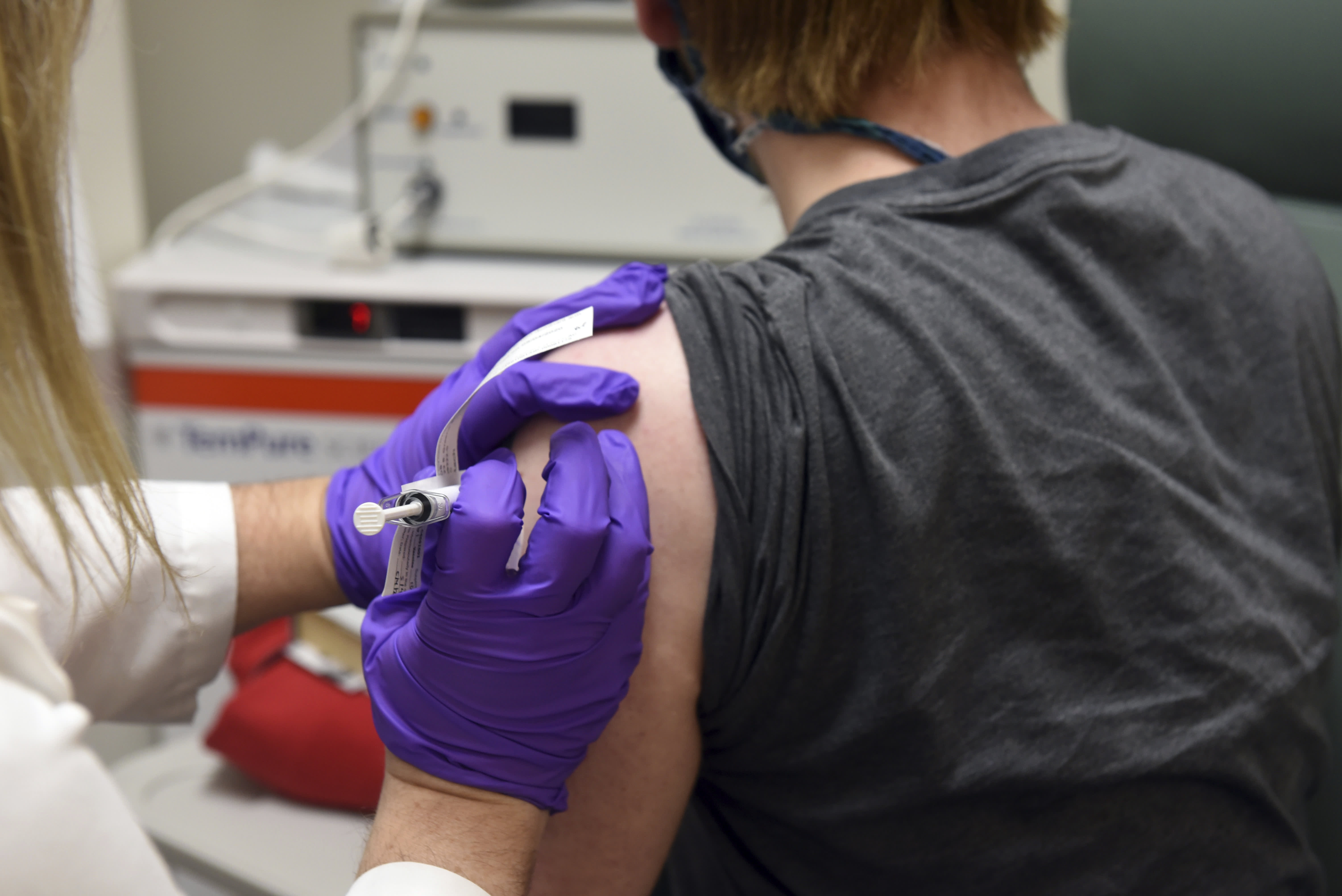
In this May 4, 2020 photo provided by the University of Maryland School of Medicine, the first patient enrolled in the Pfizer coronavirus COVID-19 vaccine clinical trial at the University of Maryland School of Medicine In Baltimore, he receives an injection.
University of Maryland School of Medicine | AP
US pharmacist Pfizer and German biotechnology BioNTech said they began their late-stage human trial for a possible coronavirus vaccine on Monday as pharmaceutical companies compete for regulatory approval before the end of the year.
The test will include up to 30,000 participants between the ages of 18 and 85 at 120 sites worldwide, the companies announced. If successful, they hope to submit it for a final regulatory review as soon as October.
The decision to start the trial reflects “our primary goal of bringing a well tolerated and highly effective vaccine to market as quickly as possible, while continuing to evaluate our other vaccine candidates as part of a differentiated portfolio of COVID-19 vaccines,” BioNTech CEO Ugur Sahin said in a statement. “Many steps have been taken towards this important milestone and we want to thank everyone involved for their extraordinary commitment.”
The companies’ experimental vaccine uses messenger ribonucleic acid, or mRNA molecules, to elicit an immune response to fight the virus. Scientists hope that mRNA, which carries genetic instructions from DNA, can be used to train the immune system to recognize and destroy the virus.
Earlier this month, the companies said that one of their four coronavirus vaccine candidates produced neutralizing antibodies, which researchers believe is necessary to create immunity to the virus, in all participants who received two of the 10 doses. or 30 micrograms.
This is a developing story. Please check for updates.
.