Doctors are worried that just before Election Day President Trump may press the U.S. Food and Drug Administration to approve a coronavirus vaccine before it is ready as an “October surprise” to win votes.
“This just can’t be allowed to happen,” Drs. Francis Collins to CNN.
He said that if FDA commissioner Dr. Stephen Hahn approves a fax based on thin evidence, “he has a lot of people to whom he should respond.”
Collins said he would be one of those people, and that would be Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Disease, so be it.
Collins said he, Fauci, and others “will certainly make a big noise about not supporting it.” [the vaccine]”if the FDA approved it too early, adding that the vaccine could not be approved” based on something other than science. ”
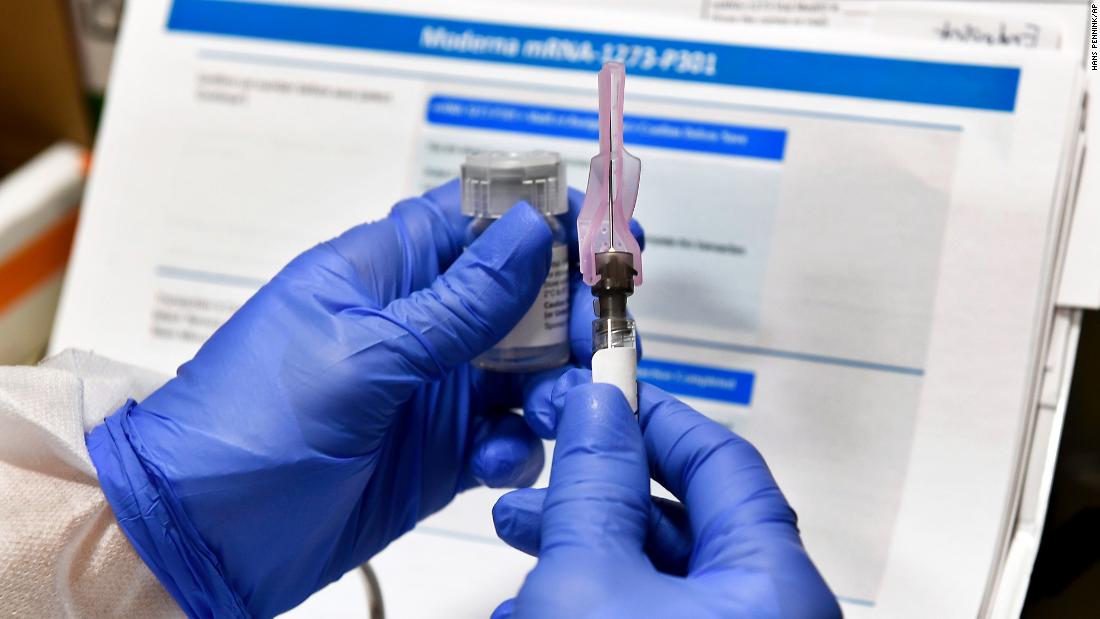
Two U.S. companies, Moderna and Pfizer, are currently conducting Phase 3 clinical trials on coronavirus vaccines, each with 30,000 research subjects who will receive several injections several weeks apart. Both tears began on July 27th.
Half of the participants will receive the vaccine and half will receive a placebo – a shot that does nothing. Then the researchers will see who gets infected with coronavirus and who does not.
There is a president for Trump who declares a product safe and effective without proof. For months, he has said that hydroxychloroquine is both, although high-quality studies have shown that it does not help patients with coronavirus and can be harmful.
“It did not go so well for HCQ did it?” Collins said, with an acronym for the drug. “Should we really be reminded of how important it is to make those decisions based on evidence?”
Collins added that he does not think Hahn, the FDA commissioner, will bow to pull pressure from Trump for a surprise in October.
“Steve Hahn is a scientist. He’s not a politician. He’s a doctor. I can not imagine him compromising his principles based on political pressure now and not. [in] November, “Collins said.
“Our decisions will be reviewed by that committee, and they will give us their guidance on every decision we make,” Hahn told Dr. Susan Bailey, president of the American Medical Association, at a Monday video briefing.
“I can assure you that once the data is available, FDA will assess it, using its established rigorous and deliberative scientific process,” he added. “We all understand that only by opening an open assessment process and relying on sound knowledge and sound data can the public, and you as providers, have confidence in the integrity of our decisions.”
Offit, who is a member of the FDA’s advisory committee on faxes, said such comments made him feel “90%” that the FDA would not allow a surprise in October.
“The reason I’m 90% and not 100% sure is that I do not trust this administration and the ability of people to pretend to please Trump,” Offit said. “This all comes down to Stephen Hahn, and Stephen Hahn is a political nominee.”
CNN reached out to a spokesman for Hahn, who did not respond to comments from doctors.
Emanuel, Offit’s co-author on the October surprise article, said Hahn had already made decisions during the pandemic that seemed politically motivated.
In March, the FDA agreed to grant emergency use authorization for hydroxychloroquine, which the agency withdrew in June. Under pressure from Trump to speed up testing, the FDA also gave the authorization of antibody tests without documentation that they worked. The agency later tightened the rules.
Emmanuel said that while he is “managed” by Hahn’s recent statements, he thinks Hahn, who has been in office for less than eight months, “has been thrown into a very, very difficult situation with intense political pressure.”
“I think Steve really wants to do the right thing here, but I’m also aware of the uniqueness of the moment and the political pressure he’s going to be under,” Emanuel added.
Offit and Emanuel said they think Hahn is aware that when he succumbs to political pressure, she and other outspoken doctors, such as Drs. Peter Hotez and Dr. William Schaffner, the airways and the internet would flood with their doubts about the vaccine.
“If there was an October surprise, I think there would be a big pushback from the professional community,” said Schaffner, a professor at Vanderbilt University Medical Center and a longtime consultant on vaccine issues at the U.S. Centers for Disease Control and Prevention.
But he adds that Trump may not care if he calls doctors like him.
“He threw out the usual manual on how to function in a pandemic,” he said.
CNN’s Andrea Kane contributed to this report.
.