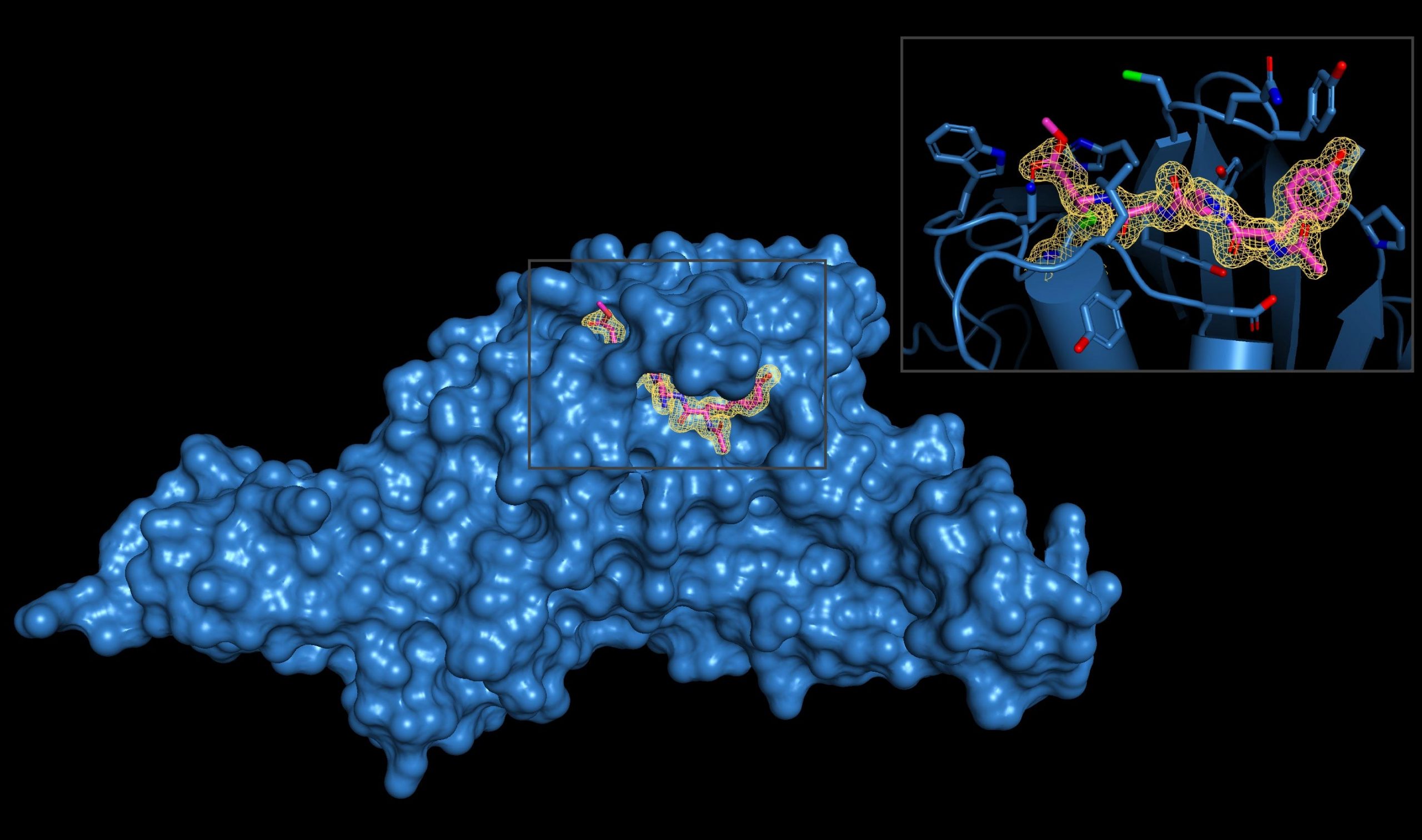

The SARS-CoV-2-PLpro enzyme is visualized by the inset of viral inhibitor interaction. Blocking the effects of the enzyme may prove beneficial in preventing coronavirus infections. Credit: Image courtesy Shawn K. Olson, PhD, Laboratory at the University of Texas Health Sciences Center at San Antonio (Joe R. and Teresa Loza’s Long School of Medicine)
Coronaviruses use enzymatic cutters to produce viruses and to disable essential immune proteins.
American and Polish scientists report in the journal October 16-20, 2020 Science progress, Put a novel logic to COVID-19 Drug Design – The virus blocks the molecule “scissors” that are used to produce the virus and to neutralize the critical human protein for the immune response.
The researchers are from the University of Texas Health Sciences Center at San Antonio (UT Health San Antonio) and the University of Science and Technology. The information collected by the American team helped Polish chemists develop two molecules that block the cutter, called an enzyme. SARS-CoV-2-PLPRO.
SARS-Covy-to-PLPO promotes infection by sensitizing and processing both viral and human proteins, says senior author Shun O. Olsen, Ph.D., Joe R. of UT. And said Teresa Loza’s associate professor of biochemistry and structural biology at Medicine’s Long School of Medicine. Health San Antonio.

PhD, Shawn K. Olson studies the SARS-Co-2-PL Pro enzyme and is collaborating with Polish chemists who have developed enzyme inhibitors. Dr. Olsen is a faculty researcher at the University of Texas Health Sciences Center at South Antonio, Joe R. and Teresa Loza’s Long School of Medicine. Credit: UT Health San Antonio
“This enzyme drives double-whammy,” Dr. Olsen said. D It. Olsen said it stimulates the release of proteins needed to replicate the virus, and it also inhibits molecules called cytokines and chemokines that signal the immune system to attack the infection.
SARS-COVI-2-PLPRO cuts human proteins ubiquitin and ISG15, which help maintain protein integrity. “The enzyme acts like nuclear scissors,” Dr. Ol. Said Olsen. “It keeps Ubicitin and ISG15 away from other proteins, making them against their normal effects.”
Dr. Olsen’s team, who recently moved from the Medical University of South Carolina to UT Health San Antonio’s School of Medicine Medicine, solved the three-dimensional structures of SARS-CAV-2-PLPRO and both inhibitory molecules. VIR 250 and VIR 251. Argo was crystallized at the National Laboratory near Chicago.
“Our collaborator, Dr. Markin Drag and his team developed inhibitors that are very efficient in blocking the activity of SARS-Co-to-PLPro, although they do not recognize the same enzymes in human cells,” said Dr. Olsen. “This is an important point: inhibitors are specific to this one viral enzyme and do not cross-react with human enzymes with similar function.”
He said the therapeutic value down the road would be crucial.
The American team also compared the similar enzymes of coronavirus, SARS-Cavi-1 and MERS from recent decades to SARS-Cavi-2-PLPRO. They learned that SARS-COVI-2-PLPRO processes ubiquitin and ISG15 more differently than its SARS-1 counterpart.
Dr. “An important question is whether he sees some of the differences we see in how the virus affects humans, if not at all,” Olsen said.
By understanding the similarities and differences of these enzymes in different coronaviruses, it may be possible to develop effective inhibitors against multiple viruses, and these inhibitors could potentially be improved in the future when other coronavirus variants come out, he said.
Reference: “Inhibition-bound SARS-CoV-2 Protein-like activity of papain profiling and crystal structures: Violetta Root, Zhongyang LV, Mykolaz Zumudzinski, Stephanie Pechet, Anti-Covid-19 Dug by Design Scott. Snipes, Farid Al Oulid, Tony T. Huang, Miklos Becks, Markin Drag and Shawn K. Olson, 16 October October 2020, Science progress.
DOI: 10.1126 / sciadv.abd4596