[ad_1]
Recent promising results for some Covid-19 vaccines and the use of new technology for two pioneers mean this is an exciting time for those working in the field, says a microbiologist.

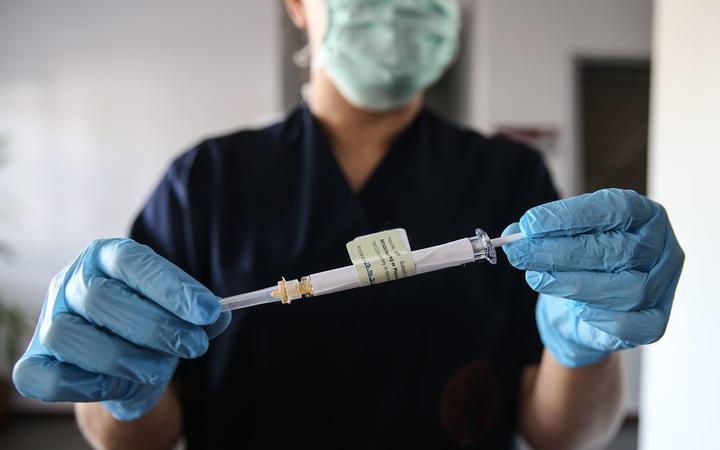
A healthcare worker holds an injection syringe of the phase 3 vaccine trial, developed by Pfizer and the German company BioNTech, at the Ibni Sina Hospital of the University of Ankara in Ankara, Turkey.
Photo: AFP
This morning, the government announced that it has reached an agreement in principle to buy doses of a Covid-19 vaccine for the entire population.
The agreement with Janssen Pharmaceutica is subject to the vaccine successfully completing clinical trials and passing regulatory approvals in New Zealand.
Last week, the government said New Zealand has an agreement with Pfizer and the German company BioNTech to buy 1.5 million vaccines, enough for 750,000 people, subject to passing all clinical trials and being approved by Medsafe.
Today, Pfizer said its final vaccine test results showed its injection had a 95 percent success rate and two months of safety data, paving the way for the drugmaker to apply for an emergency authorization in the US. USA
University of Otago clinical microbiologist David Murdoch said Nine at noon it was an exciting time for those interested in vaccines. The first vaccine reports to appear in media coverage show very high effectiveness in late-stage clinical trials involving tens of thousands of people who received a vaccine or a placebo.
However, experts were still waiting for more details on issues such as the age groups involved and how long the immunity would last.

David Murdoch.
Photo: University of Otago
He said the Pfizer and Moderna vaccines are RNA vaccines that use new technology. It involves injecting the genetic material, RNA, and this part of the genetic code will help human cells to produce the spike protein, which is the part of the virus that induces the immune reaction.
“It is a smart new approach. There is no virus really involved. It is simply an injection of RNA and it can be produced in very large volumes if successful and can potentially be adapted for different infections.”
“So there is a lot of interest and great excitement about the prospect of these vaccines.”
On the other hand, Janssen uses a technology similar to that of Astra-Zeneca called a non-replicating viral vector vaccine. They both use one type of virus, although they should not be called “live” vaccines.
“What they are is a modified virus that can be a vector to introduce those enriched proteins into the body so that the immune system can react but they do not replicate, they do not reproduce, they do not cause infection so they should be quite harmless from that point on of sight “.
Professor Murdoch said that this should make them suitable for immunosuppressed people.

A laboratory analyst is working at the Janssen Pharmaceutica headquarters in Beerse, Belgium.
Photo: AFP
When asked if one of the vaccines might be available in New Zealand before the second half of 2021, he said it was a “wait and see” matter.
‘Vaccine nationalism’ is a concern
The Pfizer and Moderna vaccines plus Janssen’s announced today by the government are ahead in development, but caution is still needed, said Professor Murdoch.
“We cannot assume that these will necessarily be the best vaccines; it turns out that they are the ones ahead in development. Obviously, the ones we’ve seen have been very promising results …”
The government targeted a portfolio of promising vaccines through the establishment of bilateral agreements. It is also contributing to the Covax acquisition initiative operated by the World Health Organization, which aims to ensure that countries around the world get equal access to vaccines.
When asked if there was a risk that a couple of large nations would dominate the procurement of vaccines in the early stages, he said it was a cause for concern.
“This vaccine nationalism that we are hearing about … is certainly a concern. Hopefully, agreements and arrangements and global collaboration will prevent that.
“Great efforts right now to try to do that with the Covax facilities and others. We hope we can combat that as much as we can.”
He said delivering the vaccines would be a great logistical exercise. A vaccine may be more suitable for a particular group of people. Pfizer’s vaccine that must be stored in very cold temperatures may not be suitable for use in the Pacific Islands.
Professor Murdoch, who has been formally supporting the University of Oxford’s Covid-19 vaccine development effort, expects an announcement on the results of his trial before the end of the year.