[ad_1]
New Zealand has agreed to purchase four Covid-19 vaccines, in volumes that are more than enough for everyone in New Zealand and also for some of our Pacific neighbors. Siouxsie Wiles on what yesterday’s announcement means and what comes next.
First a quick summary. There are many different ways to design a vaccine, using different technologies and having different advantages and disadvantages. If you need a reminder, check out the vaccine explanation Toby Morris and I put together below. More details are here. According to the New York Times Covid-19 Vaccine Tracker, there are currently 17 candidate vaccines in phase 3 clinical trials, five in limited early use, and two that have been approved by a small number of countries.

Why buy more than one vaccine?
For months, many countries have been negotiating pre-purchase agreements with companies to guarantee access to the different vaccines in development. New Zealand was no different, and it has now been revealed that we have signed pre-purchase agreements to access four different vaccines that have been developed using a variety of technologies. This is important because each technology has different advantages and disadvantages, and some vaccines will be more acceptable to some communities than others.
But there is another good reason to sign more than one agreement. When the negotiations began, there was no guarantee that all vaccines were safe and effective. The University of Queensland / CSL vaccine was recently dropped when it was discovered that some vaccinated people tested positive for HIV. They didn’t have HIV, just antibodies that reacted with the HIV test making them false positives. And that’s really bad. Without treatment, HIV is fatal. To receive treatment, people must receive a diagnosis. And that needs a reliable test, not one that returns false positives just because people have been given this vaccine. The decision was then made to abandon the vaccine, and with good reason.
If you’re curious about how people can end up with HIV-like antibodies after receiving a Covid-19 vaccine, it’s because of the way the University of Queensland researchers designed their vaccine. They created a little clamp to hold the Covid-19 spike protein in the correct shape. Unfortunately, this clamp is similar to an HIV protein, and some people also make antibodies against it.
What Covid-19 vaccines has New Zealand bought?
We have a portfolio similar to many other countries, including the US, UK, European Union, Australia, and Canada. Ours is made up of these four:
Pfizer / BioNTech mRNA vaccine
This is the one that needs to be stored at minus 70 degrees Celsius and for which the UK was the first to grant an emergency use authorization. The UK is now vaccinating people with it, as is the US It is also licensed for limited use in Kuwait, Mexico, and Singapore. Bahrain, Canada and Saudi Arabia have fully approved it, according to the New York Times vaccine tracker. New Zealand has bought enough for 750,000 people and those doses will start to arrive in the early part of next year.
Janssen / Johnson & Johnson Viral Vector Vaccine
It uses the backbone of a less harmful virus, in this case a human adenovirus, designed to contain the spike protein of the Covid-19 virus. This vaccine is still in phase 3 trials as a one-dose and two-dose vaccine. A one-dose vaccine would be a great advantage, as people would not need to return a few weeks later for a second dose. Trials of this vaccine were temporarily halted in October when someone had what was described as a “serious medical event,” but things have turned around after an investigation found no evidence that the vaccine was responsible. We have bought enough for 5 million people, but don’t expect this to happen until the end of next year or even 2022.
University of Oxford / AstraZeneca Viral Vector Vaccine
This is also in everyone else’s portfolio and is another vaccine that uses the backbone of a less harmful virus, in this case a chimpanzee adenovirus. It is also in phase 3 trials and we have ordered enough to vaccinate 3.8 million people.
Novavax protein subunit vaccine
This one is in many other portfolios as well and is still in phase 3 testing. This vaccine only uses the protein from the virus that the immune system needs to recognize, along with what is called an adjuvant that helps boost the immune system. We have ordered enough for 5.36 million people.
Why so many doses of vaccines?
If you have done the math, you will have realized that all these purchases will vaccinate more than the population of New Zealand. Again, that’s partly because we don’t know if everyone will end up getting approved. But we have also purchased vaccines for the other countries of the Kingdom of New Zealand (Tokelau, the Cook Islands and Niue) and enough to supply some of our Pacific neighbors if they wanted to. Also in yesterday’s announcement $ 75 million was earmarked to support vaccine access and deployment in the Pacific and the world, of which $ 10 million will go to the COVAX initiative, which is assembling its own portfolio of vaccines and guarantees fair and equitable access for all countries in the world.
When will we get the vaccine?
The prime minister has made it very clear that we should not expect the vaccines to become widely available in New Zealand until mid-2021. Testing of some is still ongoing, and there will only be a limited number of doses that companies can manufacture and distribute. . That manufacturing capacity is being challenged by South Africa and India who have asked the World Trade Organization to suspend intellectual property rights related to Covid-19. That would mean that manufacturing is not solely concentrated in the hands of a small number of patent holders. The pharmaceutical industry and many high-income countries strongly oppose the challenge. Surprise surprise.
To be used in New Zealand, any vaccine would also have to be approved by Medsafe. The good news is that Medsafe accepts continuous submissions of safety and efficacy data rather than sending it all at once. That means New Zealanders can rest assured that there will be no unnecessary delays in licensing vaccines if the data shows that they are safe and effective.


The big question now is, how safe and effective are the different vaccines? While the safety data looks great, the scientific community is still waiting to see all the data on how well each vaccine prevents transmission of the Covid-19 virus and prevents serious illness. That will determine how vaccines should be implemented in New Zealand and when it will be safe to open our borders again.
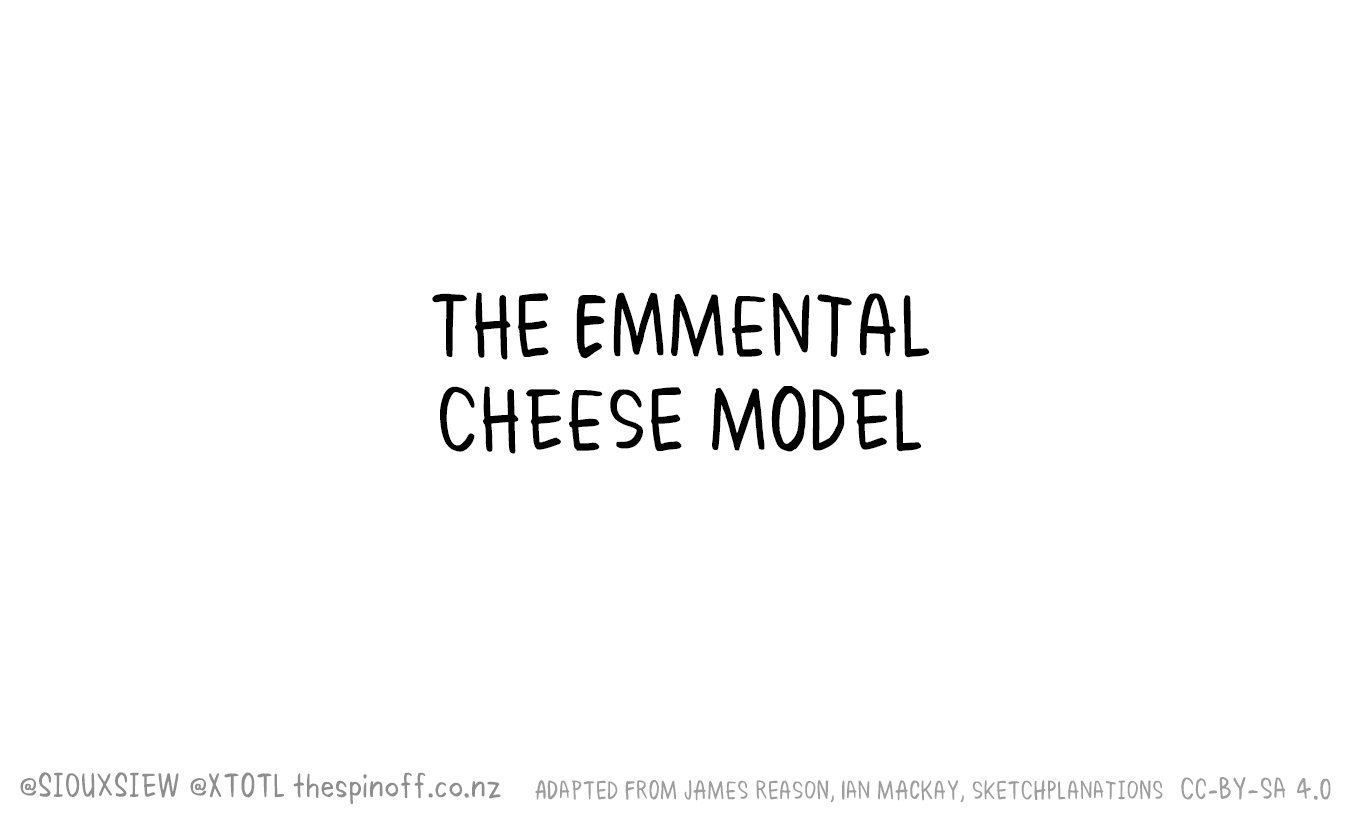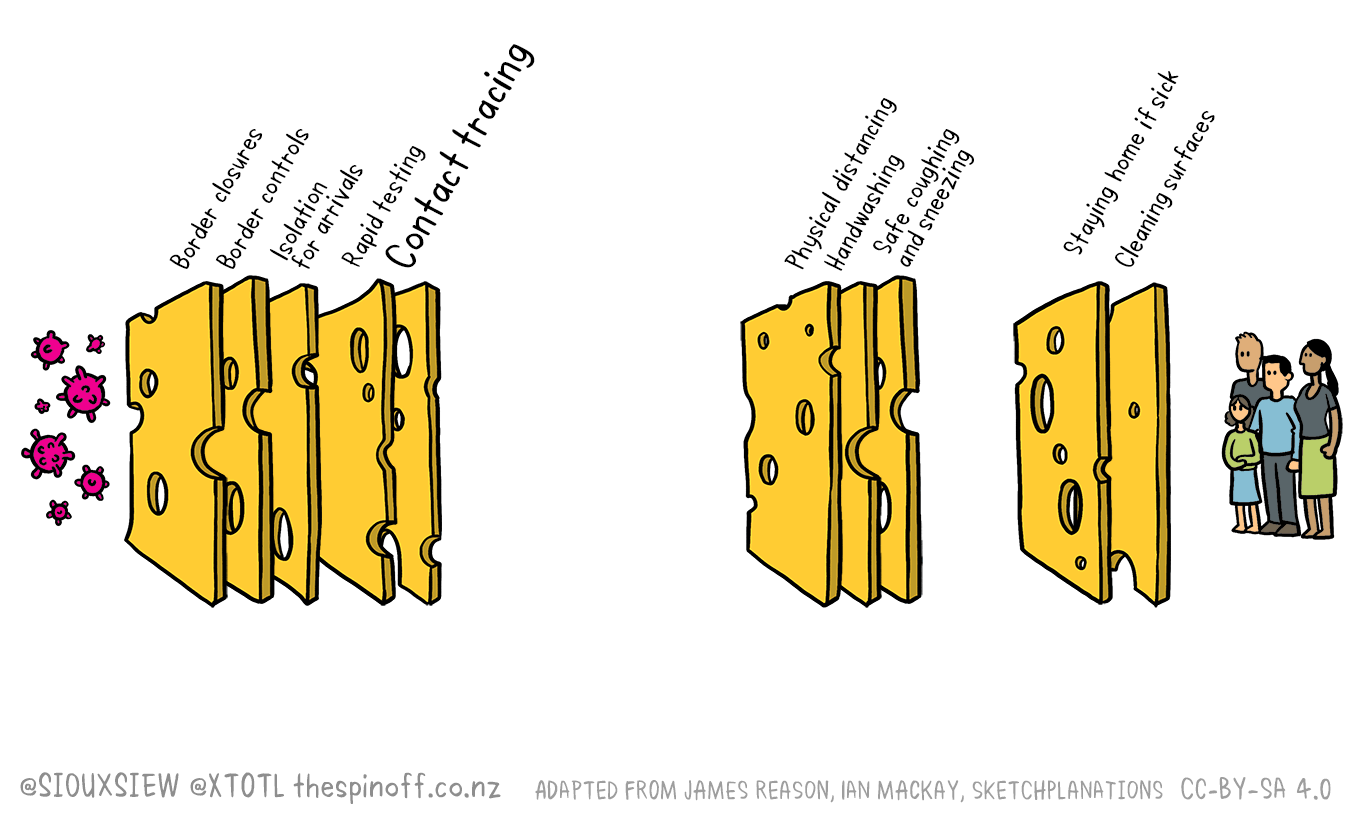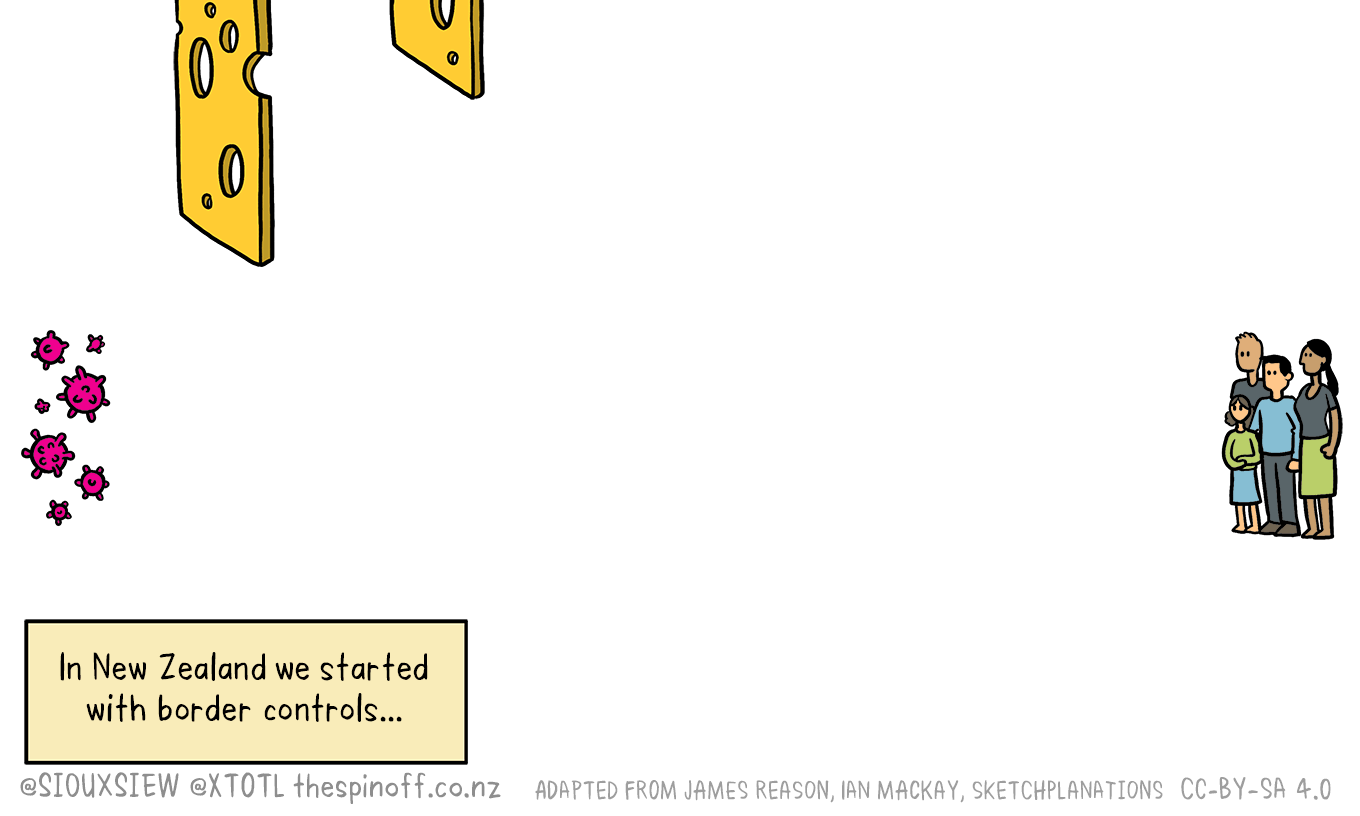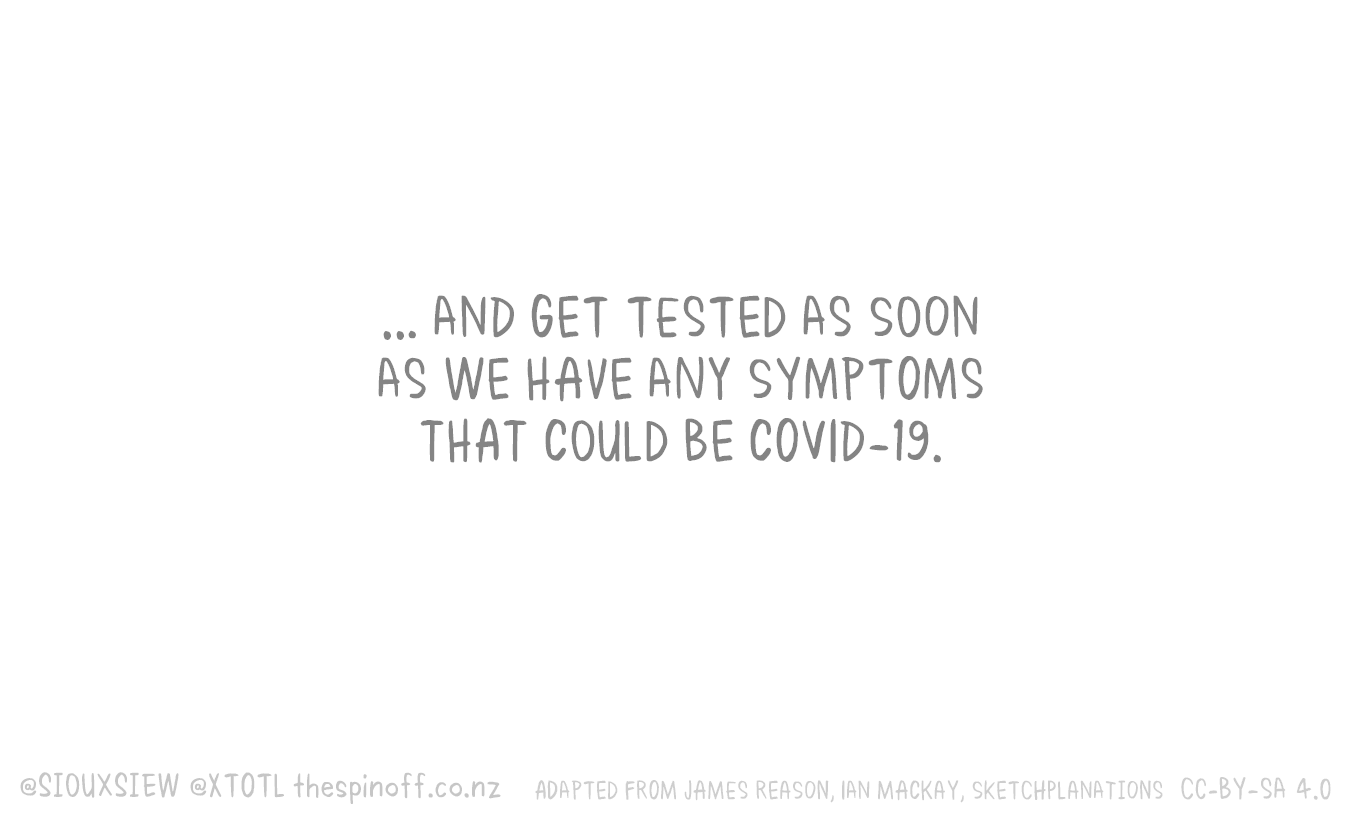
That means we will keep our Swiss / Emmental cheese model of the control measures that have served us so well so far. So if you aren’t using the Covid Tracer app yet, download it and get started. It also has a Bluetooth update, so turn it on. And don’t delay in asking for a test if you have any symptoms that could be Covid-19.
Spinoff Weekly collects the Week’s Best Stories – An Essential Guide to Modern Life in New Zealand, sent via email on Monday nights.
[ad_2]