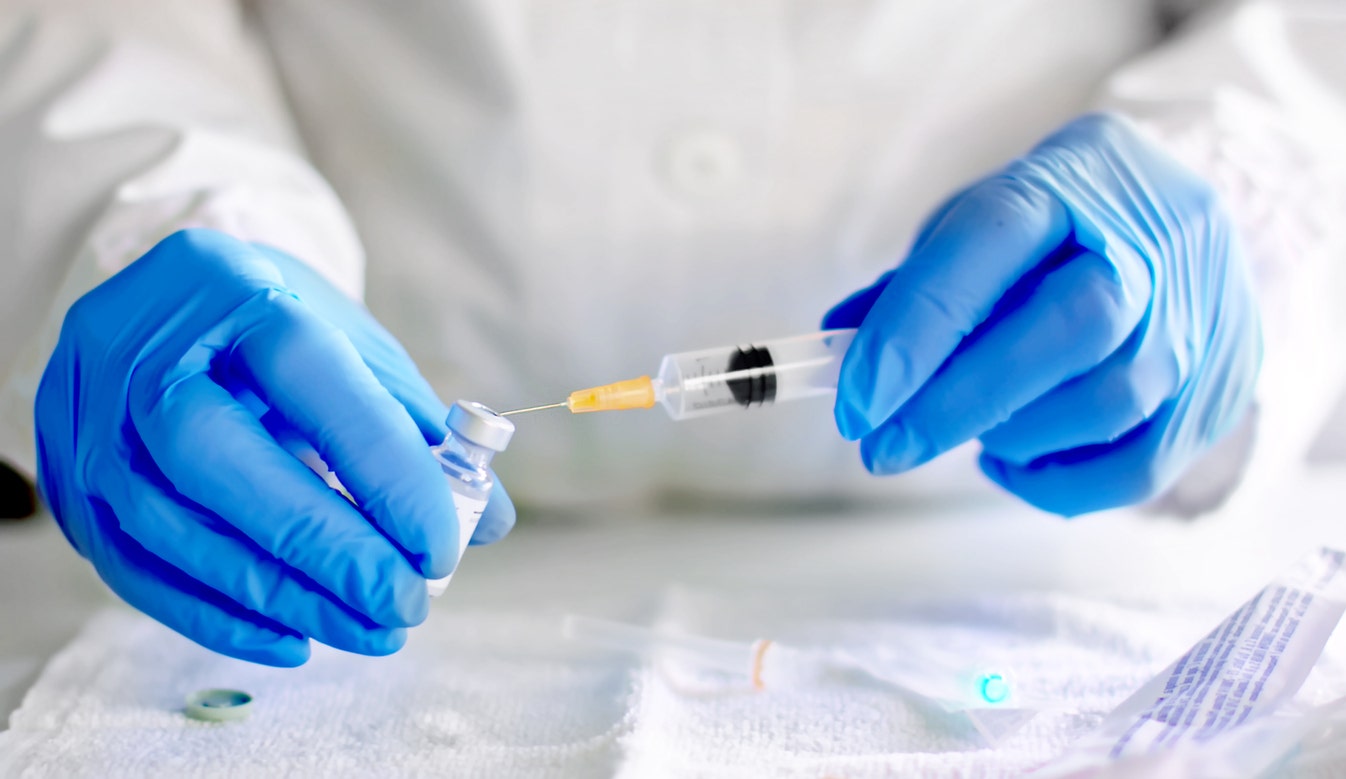
The modern biotech company said Monday it has begun dosing participants in the phase III clinical trial for its candidate for the coronavirus vaccine.
Dr. Anthony Fauci said initial clinical test results show that the vaccine candidate is safe and produces an immune response, supporting the initiation of a phase III clinical trial. Fauci is the director of the National Institute of Allergy and Infectious Diseases (NIAID) and a member of the White House Coronavirus Task Force.
CLICK HERE FOR FULL CORONAVIRUS COVERAGE
The phase III trial is called the COVE (coronavirus efficacy) study. The investigational vaccine directs cells in the body to express the tip protein of SARS-CoV-2 to elicit a broad immune response.
“Although facial covering, physical distancing, and adequate isolation and quarantine of infected people and contacts can help us mitigate the spread of SARS-CoV-2, we urgently need a safe and effective preventive vaccine to control this pandemic.” Fauci said in a press release. .
The main objective of the Phase III study is to prevent symptomatic COVID-19, with secondary objectives including preventing severe COVID-19 (patients needing hospitalization) and preventing infection with SARS-CoV-2, the virus that causes COVID-19)
In the randomized, placebo-controlled trial, volunteers will receive two intramuscular injections approximately 28 days apart from 100 micrograms of mRNA-1273 or two injections of a saline placebo.
The candidate vaccine will be evaluated at 89 clinical research sites in the United States and is expected to enroll approximately 30,000 adult volunteers who do not have the virus.
The NIH Coronavirus Prevention Network (CoVPN) will participate in conducting the trial. The network is said to bring together the expertise of existing NIAID-supported clinical research networks, and 24 of the 89 clinical research sites are part of CoVPN.
“This scientifically rigorous, randomized, placebo-controlled trial is designed to determine whether the vaccine can prevent COVID-19 and how long such protection can last,” said Fauci. The vaccine was jointly developed by Moderna, based in Massachusetts, and NIAID, part of the National Institutes of Health. Funding for the trial comes from NIAID and the Advanced Biomedical Research and Development Authority (BARDA) of the Office of the Assistant Secretary for Preparedness and Response, U.S. Department of Health and Human Services.
The vaccine efficacy trial is said to be the first implemented under Operation Warp Speed, a multi-agency collaboration led by HHS that aims to accelerate the development, manufacture, and distribution of medical countermeasures for COVID-19.
“Thanks to the leadership of President Trump and the hard work of American scientists, the investigational vaccine developed by NIH and Moderna has reached this Phase 3 trial at a record rate,” said Alex Azar, HHS Secretary. “Operation Warp Speed is supporting a portfolio of vaccines as the NIH / Moderna candidate so that, if the results of clinical trials meet the FDA gold standard, these products can reach Americans without delay in one day. “
Moderna said it remains on track to administer approximately 500 million doses per year, and possibly up to one billion doses per year, as of 2021.
CLICK HERE TO GET THE FOX NEWS APP
Fox News’ Chris Ciaccia contributed to this report.