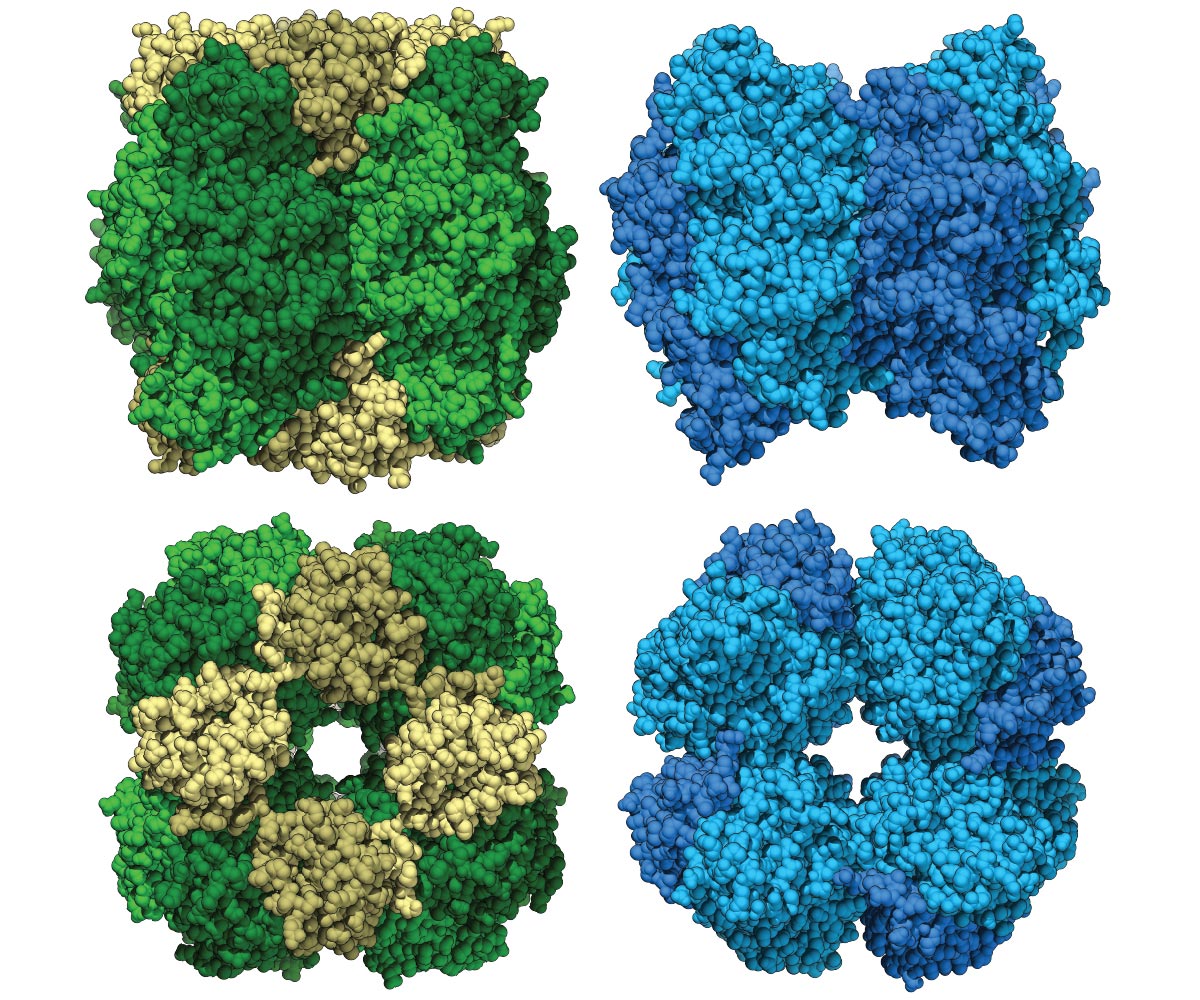

Rubisco is the most abundant enzyme on the planet. Present in plants, cyanobacteria (also known as blue-green algae) and other photosynthetic organisms, it is central to the process of carbon fixation and is the oldest carbon-fixing enzyme on Earth. Researchers at UC Davis and LBNL have now found an alternative to Rubisco in environmental samples. I compared 3D images of the Rubisco (left) form to the newly formed Form i-Prime (right). Discover how the enzyme works and use it for plant breeding. Credit: DM Banda et al, 2020
A team led by researchers at the University of California, Davis has found a missing link in the evolution of photosynthesis and carbon fixation. Over a period of more than 4.4 billion years, the newly discovered form of the plant enzyme Rubisco provides new insights into plant evolution and breeding.
Rubisco is the most abundant enzyme on the planet. Present in plants, cyanobacteria (also known as blue-green algae) and other photosynthetic organisms, it is central to the process of carbon fixation and is the oldest carbon-fixing enzyme on Earth.
“He’s the primary driver for food production, so he can take the CO2 From the atmosphere and fix that in the sugar for plants and other photosynthesis used. That is how carbon is the primary driving enzyme in life, ”said Doug Banda, a postdoctoral scholar in the laboratory of Patrick Shihni, an assistant professor of plant biology at UC Davis College College of Biological Sciences.
Forms Rubisco evolved 1.8 billion years before the Great Oxygenation Event, when cyanobacteria altered the Earth’s atmosphere by producing oxygen through photosynthesis. Rubisco’s relationship with this ancient phenomenon makes it important for scientists studying the evolution of life.
In a study published on August 31, 2020, in Plants of nature, Banda and UC Davis, researchers at UC Berkeley and LeRance Berkeley National Laboratory, report the discovery of a previously unknown relative of Form I Rubisco, which they suspect is that before the evolution of cyanobacteria, I was differentiated from the Rubisco form.
The new version, called Form I-Prime Rubisco, was found by genome sequencing of environmental samples and synthesized in the lab. Form I-Prime Rubisco gives researchers a new understanding of the structural evolution of Form I Rubisco, possibly hinting at how this enzyme changed the planet.
An invisible world
Form I Rubisco is responsible for the huge carbon fixation on Earth. But other forms of rubisco are in bacteria and in a group of microorganisms called archaea. These Rubisco variants come in a variety of shapes and sizes, and also lack small subnits. Yet they still work.
“I have something new to understand the developmental form of Rubisco, to know how small subnets evolved,” Shih said. “It’s the only form of Rubisco that we know of. It makes an octameric assembly of such large dominoes.”
Professor Jill B. Field Nuffield, co-author of the study at UC Berkeley’s Department of Earth and Planetary Sciences, unveiled the new Rubisco type after conducting a metagenomic analysis on groundwater samples. Metagenomic analysis allows researchers to examine genes and genetic sequences from an environment without culture.
“We know almost nothing about what kind of microbial life exists in the world around us, and so a huge chunk of the diversity has disappeared,” Benfield said. “The sequences we assigned to Patrick’s lab actually came from an organism that wasn’t presented in any database.”
I-Prime Rubisco successfully expressed in the lab using Banda and Shih E. coli And studied its atomic structure.
Form I Rubisco is composed of eight major large molecular subunits with eight small subunits above and below. Each part of the composition is important for photosynthesis and carbon fixation. Like Form I Rubisco, Form I-Prime Rubisco is made up of eight large subnits. However, it does not have the small subsets previously considered necessary.
“The discovery of the octameric rubisco formed without small subnits allows us to ask evolutionary questions about how life would have felt without the functionality provided by small subnits,” Banda said. “In particular, we found that form I-prime enzymes had to develop fortification interactions in the absence of small subunits, enabling structural stability at a time when the Earth’s atmosphere was rapidly changing.”
According to researchers, the form I-Prime Rubisco represents a missing link in the history of evolution. Form I Rubisco converts inorganic carbon into plant biomass, so further research on its composition and efficiency could lead to innovations in agricultural production.
Despite significant interest in engineering at ‘good’ Rubisco, there has been little success over decades of research, Shih said. “Thus, understanding how the enzyme has evolved over billions of years can provide important insights into future engineering efforts, which could ultimately improve photosynthesis productivity in crops.”
Reference: “The Bacterial Clad of the Novel Shows the Origin of Form I Rubisco” Douglas M. Banda, Jose H. Pereira, Albert K. Liu, Douglas J. Orr, Michelle Hemel, Christine Hee, Martin AJ Perry, Elizabeth Carmo-Silva, Paul L. D. Adams, Jillian F. Benfield and Patrick m. Shih, 31 August Gust 2020, Plants of nature.
DOI: 10.1038 / s41477-020-00762-4
Additional authors on the study are: UC Davis and Albert Liu at LBNL; Jose Pereira and Paul Adams, Joint Bioenergy Institute, LBNL; Christine Hee, UC Berkeley; Michelle Hemel, LBNL; And Douglas R., Martin Perry and Elizabeth Carmo-Silva, University of Leicester, UK Institute of Innovative Genomics.