
A new genomic study ranks the potential of the SARS-CoV-2 spike protein to bind to the ACE2 receptor site in 410 vertebrate animals. Old world primates and great apes, which have identical amino acids on the binding site as humans, are predicted to have a very high tendency to bind ACE2 and are likely susceptible to SARS-CoV-2 infection. Credit: Matt Verdolivo / UC Davis
- A large number of mammals can potentially be infected by SARS-CoV-2 fia has ACE2 receptors
- Some endangered species are at highest risk for SARS-CoV-2 infection via ACE2
- These species are potentially vulnerable to human SARS-CoV-2 exaggeration and should be the focus of surveillance and conservation efforts.
- The study may help identify an intermediate host as hosts for SARS-CoV-2
Humans are not the only species that have a potential threat from SARS-CoV-2, the new coronavirus that causes COVID-19, according to a new study from the University of California, Davis.
An international team of scientists used genomic analysis to compare the most important cellular receptor for the virus in humans – angiotensin-converting enzyme-2, or ACE2 – in 410 different types of vertebrates, including birds, fish, amphibians, reptiles and mammals.
ACE2 is normally found on many different types of cells and tissues, including epithelial cells in the nose, mouth and lungs. In humans 25
“class =” glossaryLink “> amino acids
of the ACE2 protein are important for the virus to bind and enter cells.The researchers used these 25 amino acids sour sequences of the ACE2 protein, and modeling of its predicted protein structure together with the SARS-CoV-2 spike protein, to evaluate how many of these amino acids are found in the ACE2 protein of different species.
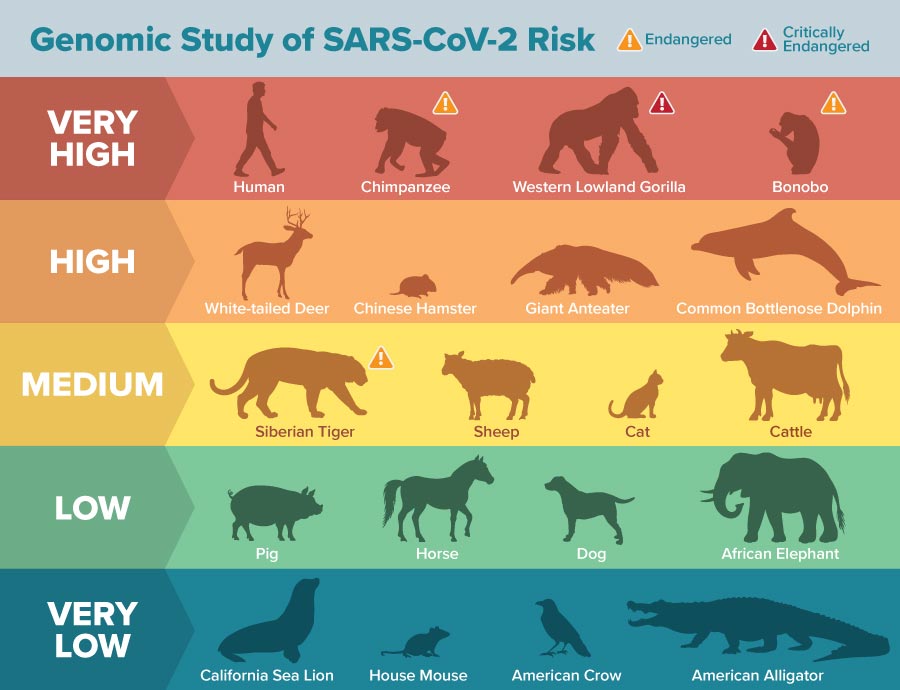
“Animals with all 25 amino acid residues corresponding to human protein are predicted to be at highest risk for SARS-CoV-2 via ACE2,” said Joana Damas, first author of the paper and a postdoctoral fellow at UC Davis. “The risk is predicted to decrease the more the ACE2-binding residues of the species differ from humans.”
About 40 percent of the species potentially susceptible to SARS-CoV-2 are classified as “endangered” by the International Union for Conservation of Nature and may be particularly vulnerable to human-to-animal transmission. The study was published Aug. 21 in the Proceedings of the National Academy of Sciences.
“The data provide an important starting point for identifying vulnerable and endangered animal populations at risk for SARS-CoV-2 infection,” said Harris Lewin, lead author for the study and a distinguished professor of evolution and ecology at UC Davis. “We hope it inspires practices that protect animal and human health during the pandemic.”
Endangered species predict to be at risk
Several critically endangered primate species, such as the Western lowland gorilla, Sumatran orangutan, and Northern white-cheeked gibbon, are predicted to be at high risk for infection by SARS-CoV-2 through their ACE2 receptor.
Other high-risk animals include marine mammals such as gray whales and bottlenose dolphins, such as Chinese hamsters.
Pets such as cats, cattle and sheep found a medium risk, and dogs, horses and barges found a low risk for ACE2 binding. How this relates to infection and disease risk should be determined by future studies, but for those species that have known data on infectivity, the correlation is high.
In documented cases of SARS-COV-2 infection in mink, cats, dogs, hamsters, lions and tigers, the virus may use ACE2 receptors, or they may use receptors other than ACE2 to access host cells. Lower propensity for binding could translate to lower propensity for infection, than lower ability for infection to spread in an animal or between once established animals.
Because of the potential for animals to contract the new human coronavirus, and vice versa, institutions including the National Zoo and the San Diego Zoo, both of which have contributed genomic material to the study, have strengthened programs to benefit both animals and to protect people.
“Zoonotic diseases and how to prevent human-to-animal transmission is not a new challenge for zoos and animal care professionals,” said co-author Klaus-Peter Koepfli, senior research scientist at Smithsonian-Mason School of Conservation and former conservation biologist with the Smithsonian Institution for Conservation Biology Institute for Species Survival and Center for Conservation Genomics. “With this new information, we can concentrate our efforts and thereby plan to keep animals and humans safe.”
The authors argue for caution against over-interpreting the predicted animal risks based on the calculation results, noting that the actual risks can only be confirmed with additional experimental data. The list of animals can be found here.
Research has shown that the immediate ancestor of SARS-CoV-2 probably originated in some kind of bat. Bats were found at very low risk to contract the new coronavirus through their ACE2 receptor, which is consistent with actual experimental data.
Whether batjes transmitted the new coronavirus directly to humans, or whether it passed through an intermediate host, is not yet known, but the study supports the idea that one or more intermediate hosts were involved. The data set researchers at zero on what species may have served as an intermediate host in the wild, and support efforts to control a future outbreak of SARS-CoV-2 infection in human and animal populations.
Reference: “Broad Host Range of SARS-CoV-2 Provided by Comparative and Structural Analysis of ACE2 in Vertebrates” by Joana Damas, Graham M. Hughes, Kathleen C. Keough, Corrie A. Painter, Nicole S. Persky, Marco Corbo , Michael Hiller, Klaus-Peter Koepfli, Andreas R. Pfenning, Huabin Zhao, Diane P. Genereux, Ross Swofford, Katherine S. Pollard, Oliver A. Ryder, Martin T. Nweeia, Kerstin Lindblad-Toh, Emma C. Teeling, Elinor K. Karlsson and Harris A. Lewin, August 21, 2020, Procedures of the National Academy of Sciences.
DOI: 10.1073 / pnas.2010146117
Additional authors on the study include: Marco Corbo, UC Davis Genome Center; Graham M. Hughes and Emma C. Teeling, University College Dublin, Ireland; Kathleen C. Keough and Katherine S. Pollard, Gladstone Institutes and UC San Francisco; Corrie A. Painter, Nicole S. Persky, Diane P. Genereux, Ross Swofford, Kerstin Lindblad-Toh and Elinor K. Karlsson, Breed Institute of MIT and Harvard, Cambridge, Mass .; Michael Hiller, Max Planck Institute of Molecular Cell Biology and Genetics, Dresden, Germany; Andreas R. Pfenning, Carnegie Mellon University, Pittsburgh; Huabin Zhao, Wuhan University, Wuhan, China; Oliver A. Ryder, San Diego Zoo Institute for Conservation Research, Escondido, and UC San Diego; Martin T. Nweeia, Harvard School of Dental Medicine, Boston, and Smithsonian Institution, Washington, DC
The research in this study was coordinated as part of the Genome 10K Organization, which includes the Bat1K, Zoonomia, the Vertebrate Genomes Project, and the Earth BioGenome Project. Genomic information for the study was also provided by the National Center for Biotechnology Information’s GenBank, the Frozen Zoo of San Diego Zoo and the Smithsonian’s Global Genome Initiative. This work was supported by the Robert and Rosabel Osborne Endowment.