[ad_1]
The UK became the first Western country to approve a Covid-19 vaccine, with the approval of its regulator. Pfizer Inc. and BioNTech SE was ahead of decisions in the US and the European Union.
The emergency authorization paves the way for the deployment of a vaccine that Pfizer and its German partner have said is 95% effective in preventing disease. The injection will be available in Great Britain. of the following weekaccording to a government statement on Wednesday.
“We can see the way out, and we can see that by the spring we are going to get through this,” Health Secretary Matt Hancock said on Sky News. In a radio interview, he added that 800,000 doses are ready to be delivered from Belgium. “This will be one of the largest civilian projects in history,” he said, with 50 hospitals preparing to administer the vaccine.
BioNTech American’s depository receipts increased 8% in early operations in Germany.
The UK had signaled that it would act swiftly to approve a vaccine, and doctors across the country were put on hold for a possible implementation. For the government, it is a opportunity to make up for missteps during the pandemic when Britain’s death toll approaches 60,000.
The UK regulator, Medicines and Health care The Product Regulatory Agency said Wednesday that the vaccine “met its strict standards for safety, quality and efficacy.” Pfizer, along with Moderna Inc. and Oxford University partner, AstraZeneca Plc, has gone ahead in an attempt to deliver coronavirus vaccines in record time.
EU application
Pfizer and BioNTech earlier this week sought regulatory clearance for their vaccine in the European Union, putting the vaccine on track for possible approval there before the end of the year. In the United States, a panel from the Food and Drug Administration will meet on December 10 to discuss the vaccine.
China has authorized its three pioneers for emergency use. Russia approved a vaccine known as Sputnik V in August, while a second inoculation was approved in October, even as the last stage of trials is still underway to establish safety and efficacy.
In late November, the British government invoked a special rule that allows its drug regulator to get ahead of the EU as the country prepares for the Brexit transition period that will conclude later this year.
Hedging bets
The UK agreed to buy 350 million doses of Covid-19 vaccines
Source: Duke Global Health Innovation Center, Bloomberg data
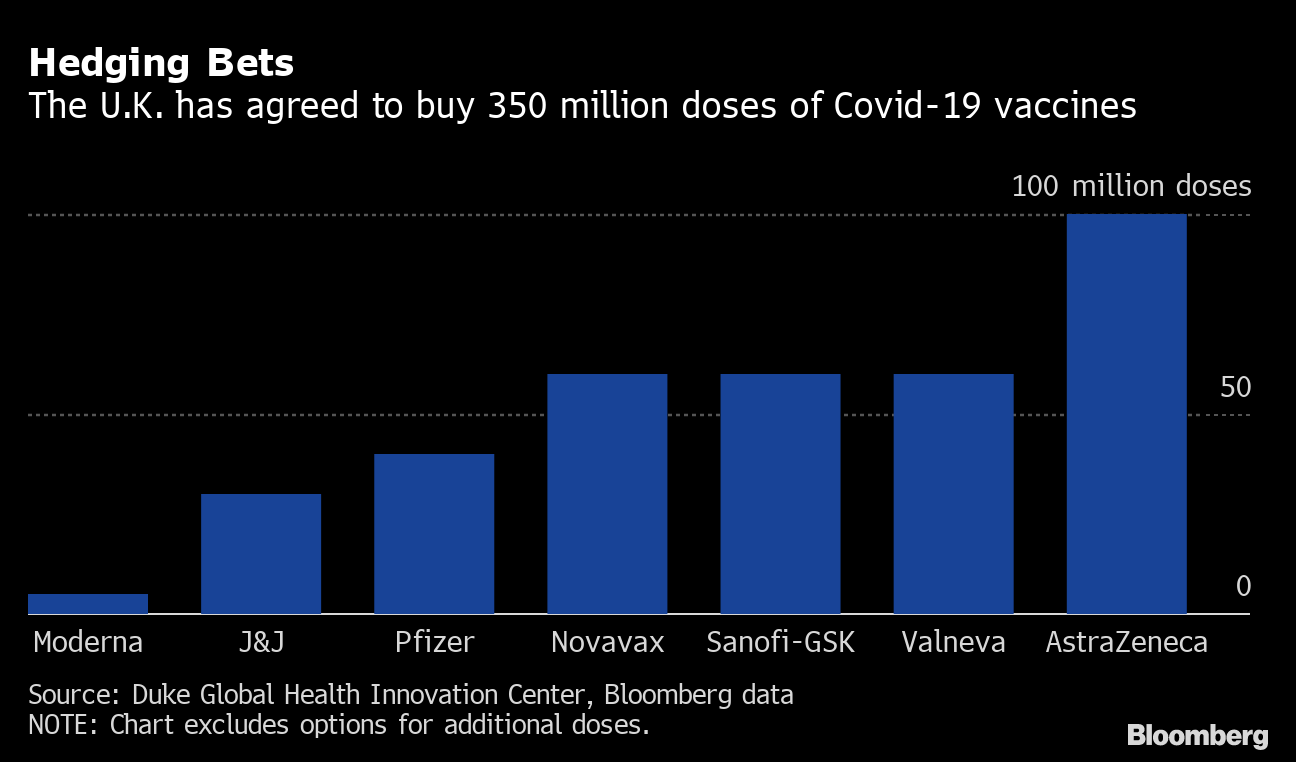
The UK still needs other vaccines to get to the finish line in order to immunize enough of its population to end the pandemic. The country has ordered enough doses of the Pfizer-BioNTech two-shot injection to inoculate 20 million people, less than a third of the population. While companies have said they can produce 1.3 billion doses next year, much of that supply is already expressed in agreements to ship hundreds of millions of injections to Europe, the United States, Japan and elsewhere.
The approval also marks the first time a messenger RNA-based vaccine has hit the market. The new technology essentially transforms the body’s cells into tiny vaccine-making machines, instructing cells to make copies of the coronavirus spike protein, which stimulates the production of protective antibodies.
The Pfizer-BioNTech photo was thrown at the head of the queue after delays in trials of the AstraZeneca-Oxford vaccine, which has also shown promising signs in preliminary results of large studies. UK partners have faced questions after acknowledging that a lower dosage level that seemed more effective resulted from a manufacturing discrepancy.
– With the assistance of Kitty Donaldson
(Updates with the comment of the secretary of health in the third paragraph, actions in the fourth paragraph)