
[ad_1]
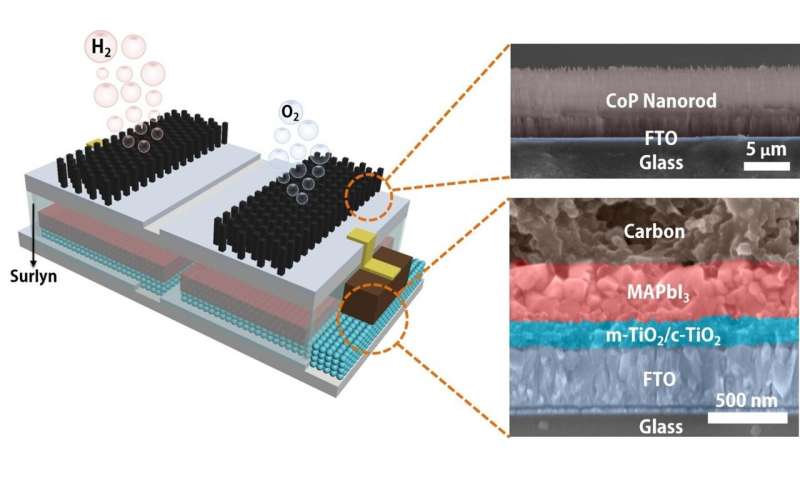
A schematic and electron microscope cross section shows the structure of an integrated catalyst powered by solar energy to divide water into hydrogen and oxygen fuel. The module developed at Rice University can be directly immersed in water to produce fuel when exposed to sunlight. Credit: Jia Liang / Rice University
Rice University researchers have created an efficient, low-cost device that divides water to produce hydrogen fuel.
The platform developed by rice materials scientist Jun Lou’s Brown School of Engineering laboratory integrates catalytic electrodes and perovskite solar cells that, when activated by sunlight, produce electricity. Current flows to catalysts that convert water to hydrogen and oxygen, with an efficiency from sunlight to hydrogen of up to 6.7%.
This type of catalysis is not new, but the lab packed a perovskite layer and electrodes in a single module that, when dropped into water and placed in sunlight, produces hydrogen without further input.
The platform presented by Lou, lead author and postdoctoral fellow Rice Jia Liang and colleagues in the American Chemical Society magazine ACS Nano It is a self-sustaining producer of fuel that, they say, should be simple to produce in bulk.
“The concept is broadly similar to an artificial leaf,” said Lou. “What we have is an integrated module that converts sunlight into electricity that generates an electrochemical reaction. It uses water and sunlight to obtain chemical fuels.”
Perovskites are cube lattice crystals known to harvest light. The most efficient perovskite solar cells produced so far reach an efficiency of over 25%, but the materials are expensive and tend to be stressed by light, humidity and heat.
“Jia has replaced the more expensive components, such as platinum, in perovskite solar cells with alternatives such as carbon,” said Lou. “That lowers the barrier to entry for commercial adoption. Integrated devices like this are promising because they create a sustainable system. This does not require any external power supply to keep the module running.”
Liang said the key component may not be perovskite but the polymer that encapsulates it, protecting the module and allowing it to submerge for long periods. “Others have developed catalytic systems that connect the solar cell out of the water with electrodes submerged with a wire,” he said. “We simplify the system by encapsulating the perovskite layer with a Surlyn (polymer) film.”
The patterned film allows sunlight to reach the solar cell while protecting it and serves as an insulator between the cells and the electrodes, Liang said.
“With an intelligent system design, you can potentially do a self-sustaining cycle,” Lou said. “Even when there is no sunlight, you can use the stored energy in the form of chemical fuel. You can put the hydrogen and oxygen products in separate tanks and incorporate another module like a fuel cell to convert those fuels into electricity.”
The researchers said they will continue to improve the encapsulation technique as well as the solar cells themselves to increase the efficiency of the modules.
New record could usher in a new era for solar energy
Jia Liang et al, a low cost, high efficiency integrated device for solar powered water splitting, ACS Nano (2020). DOI: 10.1021 / acsnano.9b09053
Provided by
Rice University
Citation:
Water division module, a perpetual energy source (2020, May 4)
Retrieved on May 4, 2020
from https://phys.org/news/2020-05-water-splitting-module-source-perpetual-energy.html
This document is subject to copyright. Apart from any fair treatment for the purpose of study or private investigation, no
part may be reproduced without written permission. The content is provided for informational purposes only.
[ad_2]