[ad_1]
In 1912, German veterinarians wondered about the case of a feverish cat with a hugely swollen belly. That is now believed to be the first reported example of the debilitating power of a coronavirus. Veterinarians were unaware at the time, but coronaviruses also gave chickens and pigs bronchitis an intestinal disease that killed almost all piglets under two weeks old.
The link between these pathogens remained hidden until the 1960s, when researchers in the United Kingdom and the United States isolated two viruses with crown-like structures that cause common colds in humans. Scientists soon noticed that the viruses identified in sick animals had the same ruffled structure, dotted with spikey protein bumps. Under electron microscopes, these viruses resembled the solar corona, prompting researchers in 1968 to coin the term coronavirus for the entire group.
It was a family of dynamic killers: dog coronaviruses could harm cats, cat coronaviruses could devastate pig intestines. The researchers thought that coronaviruses only caused mild symptoms in humans, until the outbreak of severe acute respiratory syndrome (SARS) in 2003 revealed how easily these versatile viruses could kill people.
Now, as the death toll from the COVID-19 pandemic increases, researchers strive to discover as much as possible about the biology of the latest coronavirus, called SARS-CoV-2. A killer profile is already emerging. Scientists are learning that the virus has developed a series of adaptations that make it much more deadly than the other coronaviruses that humanity has known so far. Unlike close relatives, SARS-CoV-2 can easily attack human cells at multiple sites, with the lungs and throat being the primary targets. Once inside the body, the virus makes use of a diverse arsenal of dangerous molecules. And genetic evidence suggests that it has been hiding in nature for possibly decades.
But there are many crucial unknowns about this virus, including exactly how it kills, whether it will evolve into something more or less deadly, and what it may reveal about the next outbreak in the coronavirus family.
“There will be more, either out there or in the works,” says Andrew Rambaut, who studies viral evolution at the University of Edinburgh, UK.
Bad family
Of the viruses that attack humans, coronaviruses are large. At 125 nanometers in diameter, they are also relatively large for viruses that use RNA to replicate, the group that represents the majority of emerging diseases. But coronaviruses really stand out for their genomes. With 30,000 genetic bases, coronaviruses have the largest genomes of all RNA viruses. Their genomes are more than three times as large as those of HIV and hepatitis C, and more than twice as large as those of the flu.
Coronaviruses are also one of the few RNA viruses with a genomic correction mechanism, which prevents the virus from accumulating mutations that could weaken it. That ability could be the reason why common antivirals like ribavirin, which can thwart viruses like hepatitis C, have failed to subdue SARS-CoV-2. Drugs weaken viruses by inducing mutations. But in coronaviruses, the proofreader can remove those changes.
Mutations may have their advantages for viruses. Influenza mutates up to three times more often than coronaviruses, a rate that allows it to evolve rapidly and dodge vaccines. But coronaviruses have a special trick that gives them deadly dynamism: They often recombine, exchanging fragments of their RNA with other coronaviruses. This is usually a meaningless exchange of similar parts between similar viruses. But when two distant relatives of the coronavirus end up in the same cell, recombination can lead to formidable versions that infect new cell types and jump to other species, Rambaut says.
Recombination often occurs in bats, which carry 61 viruses known to infect humans; some species harbor up to 12one. In most cases, viruses do not harm bats, and there are several theories as to why bats’ immune systems can cope with these invaders. An article published in February argues that virus-infected bat cells quickly release a signal that allows them to harbor the virus without killing it.2.
Estimates for the birth of the first coronavirus vary widely, from 10,000 years ago to 300 million years ago. Scientists are now aware of dozens of strains3, seven of which infect humans. Among the four that cause common colds, two (OC43 and HKU1) come from rodents and the other two (229E and NL63) from bats. All three that cause serious illness: SARS-CoV (the cause of SARS), Middle East respiratory syndrome MERS-CoV, and SARS-CoV-2, all come from bats. But scientists think that there is usually an intermediary: an animal infected by bats that carries the virus to humans. With SARS, the intermediary is believed to be civet cats, which are sold in live animal markets in China.
The origin of SARS-CoV-2 remains an open question (see “Family of Assassins”). The virus shares 96% of its genetic material with a virus found in a bat in a cave in Yunnan, China.4 4 – A compelling argument that it comes from bats, researchers say. But there is a crucial difference. The coronavirus peak proteins have a unit called the receptor binding domain, which is critical to their success in entering human cells. The SARS-CoV-2 binding domain is particularly efficient, and differs in important ways from the Yunnan bat virus, which does not appear to infect humans.5 5.
To complicate matters, a scaly anteater named pangolin appeared with a coronavirus that had a receptor-binding domain almost identical to the human version. But the rest of the coronavirus was only 90% genetically similar, so some researchers suspect that the pangolin was not the intermediary.5 5. The fact that both mutations and recombinations work complicates efforts to draw a family tree.
But studies published in recent months, which have not yet been peer-reviewed, suggest that SARS-CoV-2, or a very similar ancestor, has been hiding in some animals for decades. According to an article published online in March6 6, the coronavirus lineage that led to SARS-CoV-2 separated more than 140 years ago from the closely related one seen today in pangolins. Then, sometime in the last 40–70 years, the ancestors of SARS-CoV-2 separated from the bat version, which subsequently lost the effective receptor binding domain that was present in its ancestors (and remains in SARS -CoV-2). A study published on April 21 yielded very similar results using a different dating method.7 7.
These results suggest a long family history, with many bat coronavirus branches and possibly pangolins that carry the same deadly receptor binding domain as SARS-CoV-2, including some that might have similar capabilities to cause a pandemic, Rasmus says. Nielsen, an evolutionary biologist at the University of California, Berkeley, and co-author of the second study. “There is a need for continued vigilance and increased vigilance towards the emergence of new viral strains by zoonotic transfer,” he says.
Two open doors
Although known human coronaviruses can infect many types of cells, they all primarily cause respiratory infections. The difference is that the four that cause common colds easily attack the upper respiratory tract, while MERS-CoV and SARS-CoV have a harder time staying there, but are more successful in infecting cells in the lungs.
The SARS-CoV-2, unfortunately, can do both very efficiently. That gives him two places to settle, says Shu-Yuan Xiao, a pathologist at the University of Chicago, Illinois. The cough of a neighbor who sends ten viral particles in his direction might be enough to start an infection in the throat, but the hair-shaped cilia found that they are likely to do their job and kill the invaders. If the neighbor is closer and coughs 100 particles towards you, the virus could reach the lungs, says Xiao.
These varying capabilities could explain why people with COVID-19 have such different experiences. The virus can start in the throat or nose, causing a cough and interrupting taste and smell, and then ends there. Or it could reach the lungs and weaken that organ. Stanley Perlman, an immunologist at the University of Iowa in Iowa City, who studies coronaviruses, does not know how it gets there, if it moves cell by cell or somehow washes away.
Clemens-Martin Wendtner, an infectious disease doctor at the Schwabing Clinic in Munich in Germany, says it could be a problem with the immune system that allows the virus to leak into the lungs. Most infected people create neutralizing antibodies that are designed by the immune system to bind with the virus and prevent it from entering the cell. But some people seem unable to do them, says Wendtner. That may be why some recover after a week of mild symptoms, while others suffer from late-onset lung disease. But the virus can also bypass cells in the throat and go directly to the lungs. Then, patients can get pneumonia without the usual mild symptoms, such as a cough or a mild fever that would otherwise come first, Wendtner says. Having these two infection points means that SARS-CoV-2 can mix the transmissibility of coronaviruses of the common cold with the lethality of MERS-CoV and SARS-CoV. “It is an unfortunate and dangerous combination of this coronavirus strain,” he says.
The virus’s ability to actively infect and reproduce in the upper respiratory tract was somewhat surprising, given that its close genetic relative, SARS-CoV, lacks that ability. Last month, Wendtner published results8 from experiments in which his team was able to grow virus from the throat of nine people with COVID-19, showing that the virus is actively reproducing and infectious there. That explains a crucial difference between close relatives. SARS-CoV-2 can dump viral particles from the throat into saliva even before symptoms begin, and these can easily pass from person to person. SARS-CoV was much less effective in making that jump, passing only when symptoms were in full swing, making it easier to contain.
These differences have led to some confusion over the case fatality of SARS-CoV-2. Some media and expert reports describe it as less deadly than SARS-CoV because it kills about 1% of the people it infects, while SARS-CoV killed about ten times that rate. But Perlman says that’s the wrong way to look at it. SARS-CoV-2 is much better at infecting people, but many infections do not progress to the lungs. “Once it reaches the lungs, it’s probably just as deadly,” he says.
What it does when it reaches the lungs is similar in some ways to what respiratory viruses do, although much remains to be known. Like SARS-CoV and influenza, it infects and destroys the alveoli, the tiny sacs in the lungs that carry oxygen to the bloodstream. As the cellular barrier that divides these sacs from the blood vessels breaks down, the fluid in the vessels leaks, preventing oxygen from reaching the blood. Other cells, including white blood cells, further block the airways. A robust immune response will clear all this up in some patients, but overreaction of the immune system can worsen tissue damage. If the inflammation and tissue damage are too severe, the lungs never recover and the person dies or is left with scarred lungs, Xiao says. “From a pathological point of view, we don’t see much uniqueness here.”
And as with SARS-CoV, MERS-CoV, and animal coronaviruses, the damage does not stop with the lungs. A SARS-CoV-2 infection can trigger an excessive immune response known as a cytokine storm, which can lead to multiple organ failure and death. The virus can also infect the intestines, heart, blood, sperm (like MERS-CoV), the eye, and possibly the brain. Damage to the kidney, liver, and spleen seen in people with COVID-19 suggests that the virus can be carried in the blood and infect various organs or tissues, says Guan Wei-jie, a pulmonologist at the Guangzhou Institute of Respiratory Health in Guangzhou. Medical. University, China, an institution praised for its role in the fight against SARS and COVID-19. The virus could infect various organs or tissues wherever the blood supply arrives, Guan says.
But although the genetic material of the virus is appearing in these various tissues, it remains unclear whether the damage is being caused by the virus or by a cytokine storm, Wendtner says. “Autopsies are being performed at our center. More data will come soon, ”he says.
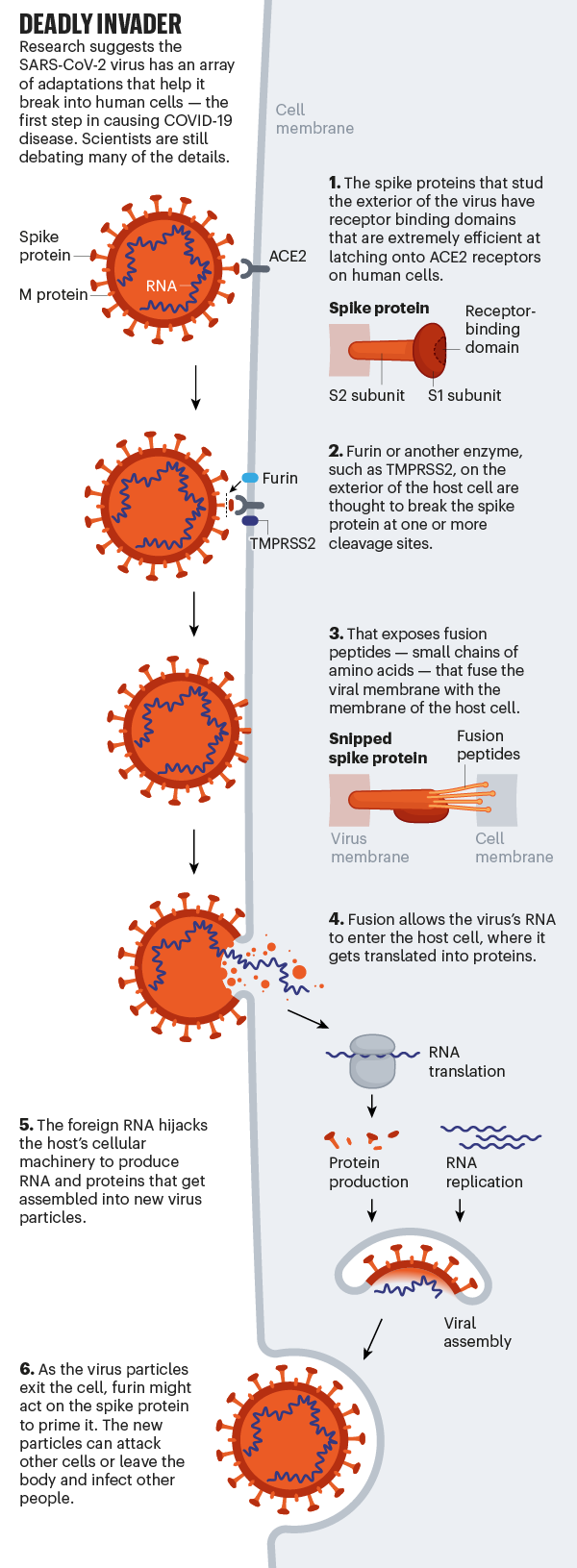
Whether it infects the throat or lungs, SARS-Cov-2 breaks the protective membrane of host cells using its spiky proteins (see “Deadly Invader”). First, the receptor binding domain of the protein binds to a receptor called ACE2, which is located on the surface of the host cell. ACE2 is expressed throughout the body in the lining of the arteries and veins that run through all the organs, but is particularly dense in the cells that line the alveoli and small intestine.
Although the exact mechanisms remain unknown, evidence suggests that after the virus attaches to itself, the host cell cuts the spike protein at one of its dedicated ‘cleavage sites’, exposing fusion peptides, small chains of amino acids. that help open up the host cell. membrane so that the virus membrane can fuse with it. Once the invader’s genetic material enters the cell, the virus appropriates the host’s molecular machinery to produce new viral particles. That progeny then leaves the cell to go and infect others.
Power spikes
SARS-CoV-2 is uniquely equipped to force entry into cells. Both SARS-CoV and SARS-CoV-2 bind to ACE2, but the receptor binding domain of SARS-CoV-2 is a particularly tight fit. It is 10-20 times more likely to bind ACE2 than SARS-CoV9 9. Wendtner says that SARS-CoV-2 is so good at infecting the upper respiratory tract that there could even be a second receptor that the virus could use to launch its attack.
Even more concerning is the fact that SARS-COV-2 appears to make use of the host furin enzyme to cleave the viral peak protein. This is concerning, the researchers say, because furin is abundant in the respiratory tract and is found throughout the body. It is used by other formidable viruses, such as HIV, influenza, dengue, and Ebola, to enter cells. In contrast, the cleavage molecules used by SARS-CoV are much less common and not as effective.
Scientists think that the involvement of furin could explain why SARS-CoV-2 is so good for jumping from cell to cell, person to person, and possibly animal to human. Robert Garry, a virologist at Tulane University in New Orleans, Louisiana, estimates that it gives SARS-CoV-2 a probability 100 to 1,000 times greater than SARS-CoV of penetrating deep into the lungs. “When I saw that SARS-CoV-2 had that cleavage site, I didn’t sleep very well that night,” he says.
The mystery is where the genetic instructions for this particular cleavage site come from. Although the virus probably acquired them by recombination, this particular configuration has never been found in any other coronavirus in any species. Determining its origin could be the last piece of the puzzle that will determine which animal was the springboard that allowed the virus to reach humans.
End of the game
Some researchers hope that the virus will weaken over time through a series of mutations that adapt it to persist in humans. By this logic, it would be less deadly and more likely to spread. But researchers have yet to find any signs of such a weakening, likely due to the virus’s efficient genetic repair mechanism. “The COVID-19 virus genome is very stable and I don’t see any pathogenicity changes caused by the virus mutation,” says Guo Deyin, who investigates coronaviruses at Sun Yat-sen University in Guangzhou.
Rambaut, too, doubts that the virus will become milder over time and save its host. “It doesn’t work that way,” he says. As long as you can successfully infect new cells, reproduce, and transmit to new ones, it doesn’t matter if it hurts the host, he says.
But others think that there is a possibility of a better result. It could give people antibodies that will offer at least partial protection, says Klaus Stöhr, who headed the SARS research and epidemiology division of the World Health Organization. Stöhr says that immunity will not be perfect: People who are reinfected will still develop minor symptoms, as they do now from the common cold, and there will be rare examples of serious illness. But the virus’s proofreading mechanism means it won’t mutate quickly, and infected people will retain strong protection, he says.
“By far the most likely scenario is that the virus will continue to spread and infect the majority of the world’s population in a relatively short period of time,” says Stöhr, which means one or two years. “Later, the virus will continue to spread in the human population, probably forever.” Like the four generally mild human coronaviruses, SARS-CoV-2 would circulate constantly and primarily cause mild upper respiratory tract infections, Stöhr says. For that reason, he adds, vaccines will not be necessary.
Some previous studies support this argument. One10 showed that when people were inoculated with the common cold coronavirus 229E, their antibody levels peaked two weeks later and only increased slightly after one year. That did not prevent infections a year later, but subsequent infections caused few symptoms, if any, and a shorter period of viral transmission.
The coronavirus OC43 offers a model of where this pandemic could go. That virus also gives humans common colds, but genetic research from the University of Leuven in Belgium suggests that OC43 may have been a killer in the past.eleven. That study indicates that OC43 spread to humans around 1890 from cows, which they obtained from mice. Scientists suggest that OC43 was responsible for a pandemic that killed more than a million people worldwide in 1889-1890, an outbreak previously attributed to influenza. Today, OC43 continues to circulate widely, and it could be that continued exposure to the virus keeps the vast majority of people immune to it.
But even if that process made OC43 less deadly, it’s still unclear if something similar would happen with SARS-CoV-2. A study in monkeys showed that they retained antibodies to SARS-CoV-2, but the researchers only reported the first 28 days after infection, making it unclear how long the immunity lasted.12. Antibody concentrations against SARS-CoV also decreased significantly over a period of two to three years.13. Whether these low levels would be sufficient to prevent infection or reduce severity has not been proven. Cats, cows, dogs, and chickens do not appear to be immune to the sometimes deadly coronaviruses that infect them, leaving vets over the years to search for vaccines. Despite all the questions about whether people retain any immunity to SARS-CoV-2, some countries are promoting the idea of giving survivors ‘immunity passports’ to allow them to leave without fear of becoming infected or infecting others.
Many scientists reserve judgment on whether tamer coronaviruses were ever as virulent as SARS-CoV-2. People like to think that “the other coronaviruses were terrible and mild,” says Perlman. “That is an optimistic way of thinking about what is happening now, but we have no evidence.”