[ad_1]
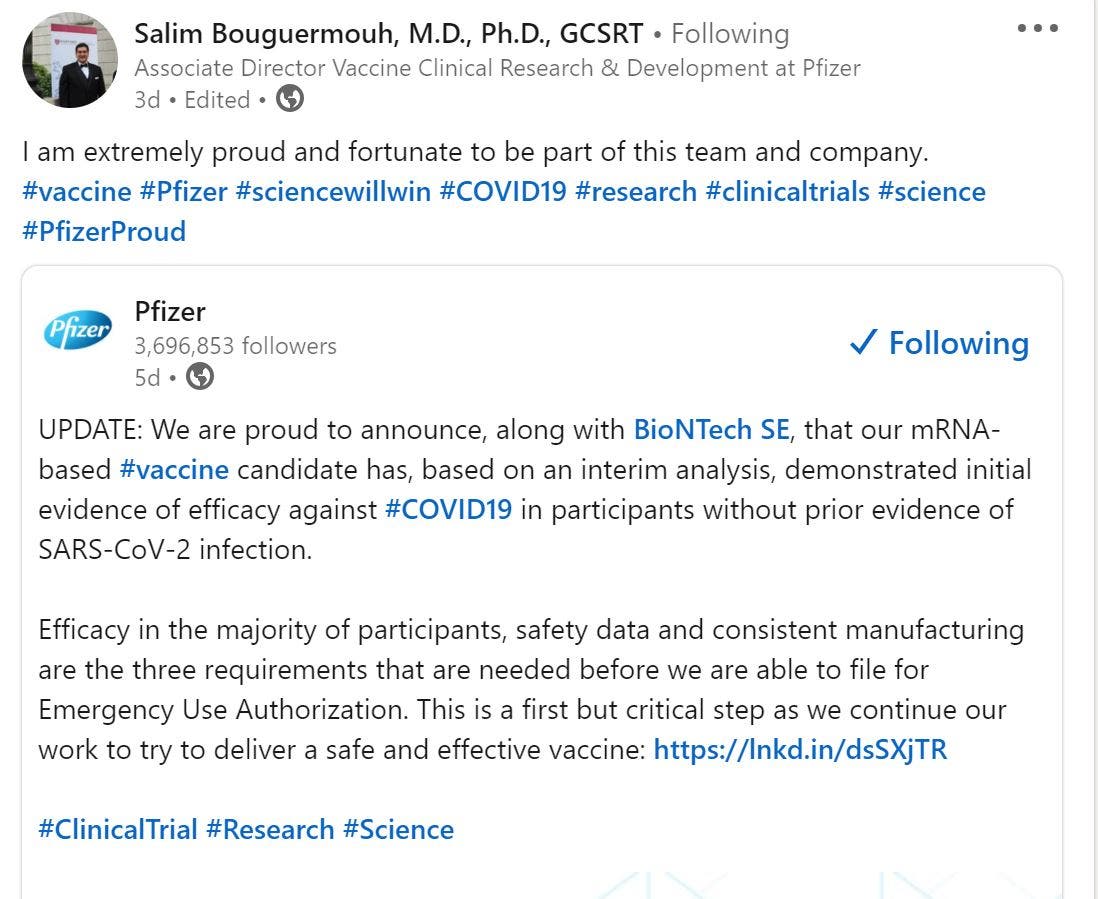
The whole world is waiting for new news about the vaccine from the American laboratories “Pfizer” and “Biontech” German, which have announced that it will be developed with an efficiency of up to 90%.
Faced with this news, sources revealed that the list of medical personnel of the US global pharmaceutical manufacturer “Pfizer” included the Algerian researcher, Salim Boukarmouh, who is the deputy director responsible for clinical research and vaccine development at the company.
And Professor Boukarmouh announced, through his official account on the “Linkedin” website, on the matter, saying that “science will win.”
He also confirmed that he is very proud to be part of the team leading the clinical trials of the long-awaited vaccine.
Who is the Arab teacher?
It should be noted that Professor Boukarmouh has spent many years conducting clinical and translational research in several major laboratories around the world, most notably at the University of “Poitiers” in France, the University of “Montreal” in Canada, and the “Massachusetts Institute” for technology research in Singapore.
The Algerian researcher published more than 10 reference articles, which were presented in more than 11 international conferences.
As for his beginnings, he was in the specialty of medicine at the University of Algiers in 1996-1987, after which he obtained a diploma in “Specialized Medical Studies in Microbiology and Microbiology” at the University of Algiers in 1998-2001.
After that, he moved to Canada, where he joined the Canadian Medical Council to evaluate the MCCEE exams in 2007.
From 2010 to 2012, Bougarmouh participated in the Massachusetts Institute of Technology for Research and Technology in Singapore, specializing in Immunology and Microbiology.
Until he decided to settle in the United States, where he enrolled at Harvard University and obtained international certificates in 2014-2015.
The Arab world held many positions until he reached the position of Deputy Director of Vaccine Research and Development at Pfizer International Laboratories in 2017.
Hope looms … and it’s soon
It is noteworthy that the American laboratories, “Pfizer” and German “Biontec”, had announced a few days ago, a vaccine that had shown an efficacy of 90%, according to preliminary results for the third phase of trials of experiments that involved more of 40,000 people.
They also explained that protection against the coronavirus is achieved at a high rate 28 days after taking the first dose, and that it is completed only one week after the second dose.
The company is awaiting the safety data, but could apply for an emergency clearance with regulators earlier this month.
In turn, Dr. Albert Burla, president and CEO of Pfizer at the time, described that day as the great day for science and humanity and said that “the first set of results from the third phase of the trial of the Covid-19 vaccine provides preliminary evidence of the ability of our vaccine to prevent corona. ” .
He also added: “We have reached this milestone in our vaccine development program at a time when the world needs it most, with record rates of infection on record.”
Furthermore, the World Health Organization praised this achievement, noting that the vaccine is promising and promising.
