[ad_1]
The application also includes safety data for 100 children between the ages of 12 and 15, and the company said 45 percent of the participants in the US trials are between 56 and 85 years old.
The Secretary of Health of the United States, Alex Azar, told the network "CBS" Newsletter if the data is solid, "We may have literally weeks before a license for a vaccine that is 95 percent effective.".
The companies expect the Food and Drug Administration to grant approval for emergency use in mid-December.
They said they would start shipping the doses immediately, and Pfizer said it expects to have 50 million doses of vaccines ready this year, enough to protect 25 million people.
“>
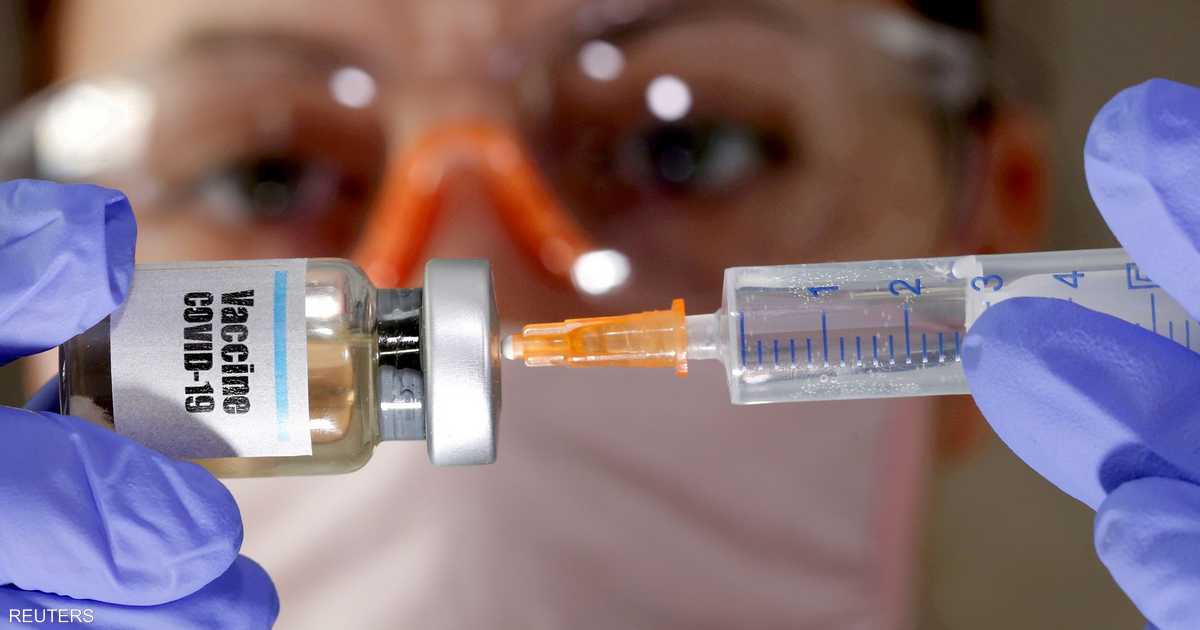
Pfizer has submitted a request to interested health authorities in the United States to approve the emergency use of the Covid-19 vaccine, which is the first request of its kind in an important step to provide protection against the emerging corona virus.
The application to the US Food and Drug Administration comes days after Pfizer and its German partner Biontech announced the results of final trials, which showed that the vaccine is effective in preventing Covid-19 by 95%. percent without any security problem.
Pfizer shares rose 1.6 percent and Biontech shares rose 6 percent after news of the possibility of a vaccine being available soon, fueling hopes of ending a pandemic that has killed to more than a quarter of a million people in the United States and more than 1.3 million worldwide.
The application also includes safety data for 100 children between the ages of 12 and 15, and the company said 45 percent of the participants in the US trials are between 56 and 85 years old.
US Health Secretary Alex Azar told CBS News that if the data is strong, “we could have literally weeks before we get a license for a vaccine that is 95 percent effective.”
The companies expect the Food and Drug Administration to grant approval for emergency use in mid-December.
They said they would start shipping the doses immediately, and Pfizer said it expects to have 50 million doses of vaccines ready this year, enough to protect 25 million people.
[ad_2]