[ad_1]

On the 26th, when vaccination against the new coronavirus (Corona 19) infection began, medical staff at the Dobong-gu Public Health Center in Seoul hold the AstraZeneca (AZ) vaccine in a syringe. Central photo
Kang-rip Kim, head of the Ministry of Food and Drug Safety, said on the 26th: “You may feel relieved”, when the AstraZeneca vaccination of the new coronavirus infection (Corona 19) began.
Kim said in an interview with KBS Radio ‘Choi Management’s Strongest Breakthrough’, “The results of (countries) that inoculated this vaccine are reported to us. “There are currently no reports of safety-related adverse events, even in large-scale outcomes.”
Director Kim said the AstraZeneca vaccine was “approved by the government.” “Going through the national approval process, we thoroughly verify the safety and efficacy, so that it is suitable not only for Korean standards but also for international standards. He stressed that it was also recognized in the three-person consultation process.
Regarding the ‘triple advisory system’, “we introduced a new professional verification system at the work level, instead of first, and we went through the central pharmacist review committee, and finally, the final review committee in which external experts participate.
“It is the first vaccine to be vaccinated in Korea, so it is very important that people trust the vaccine and get it voluntarily.”
Director Kim also said, “When we look at vaccines, we look at two things. One is, is it safe? The second is whether it works.” “Vaccines usually have some adverse reactions to trigger an immune response in the body. “There is no vaccine on the planet that is completely free from adverse reactions to any vaccine.” “However, it was verified if there was such an unusual situation, that it was much greater than the risk when the risk was not met when the vaccine was administered because it was so serious. No such information was reported in the clinical trial. There have been no reports. of unusual adverse reactions “.
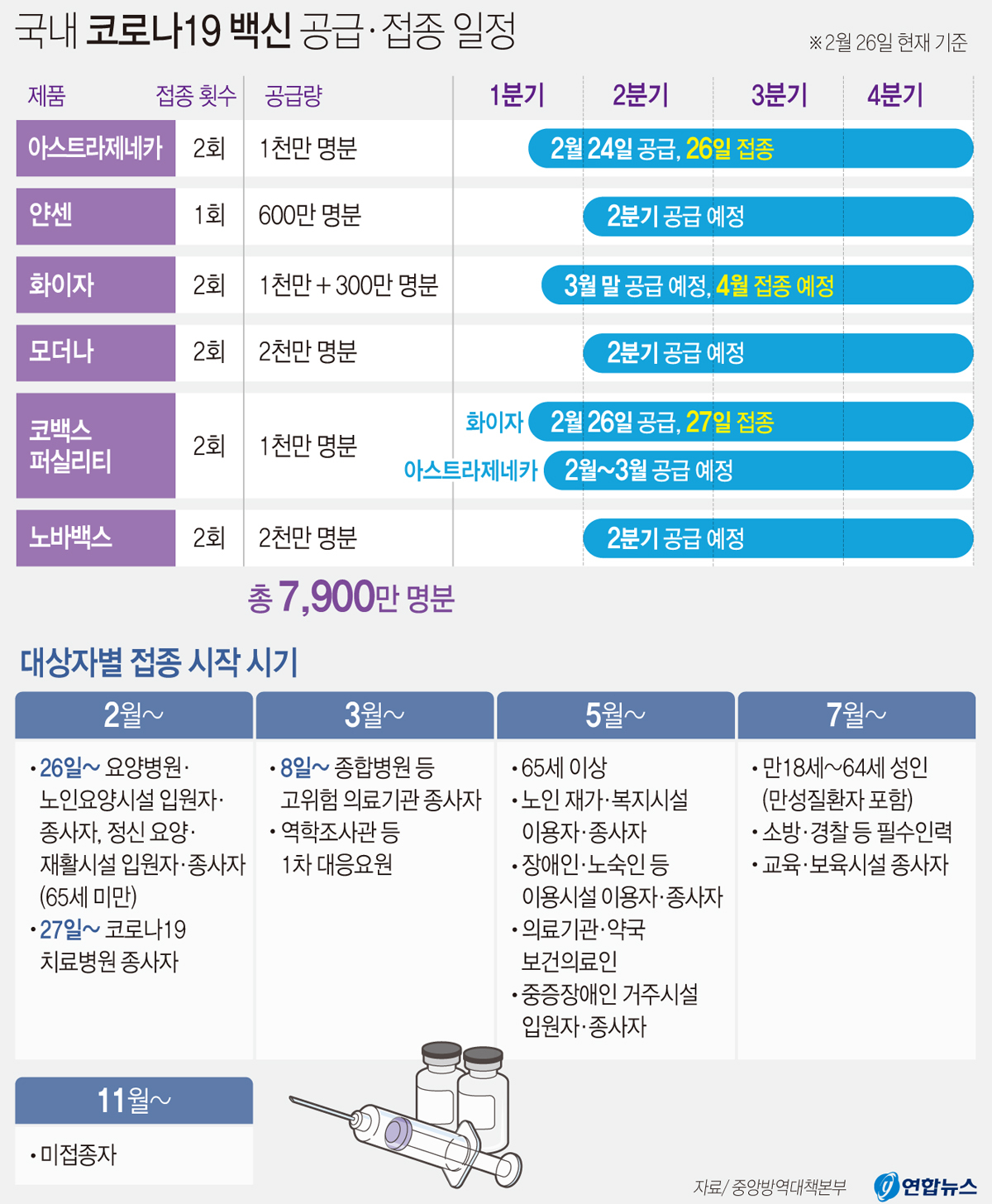
Supply of national Corona 19 vaccines and vaccination schedule. Yunhap news
Starting at 9 a.m. that day, the first domestic vaccination against Corona 19 began in various parts of the country. It has been a year and 37 days since January 20 last year when the first 19th crown was confirmed in Korea.
Vaccination against AstraZeneca began at the same time in 1,915 locations, including public health centers and nursing hospitals across the country. The targets for vaccination on this day are 5,266 nursing facility workers and residents under 65, and nursing hospital residents and workers.
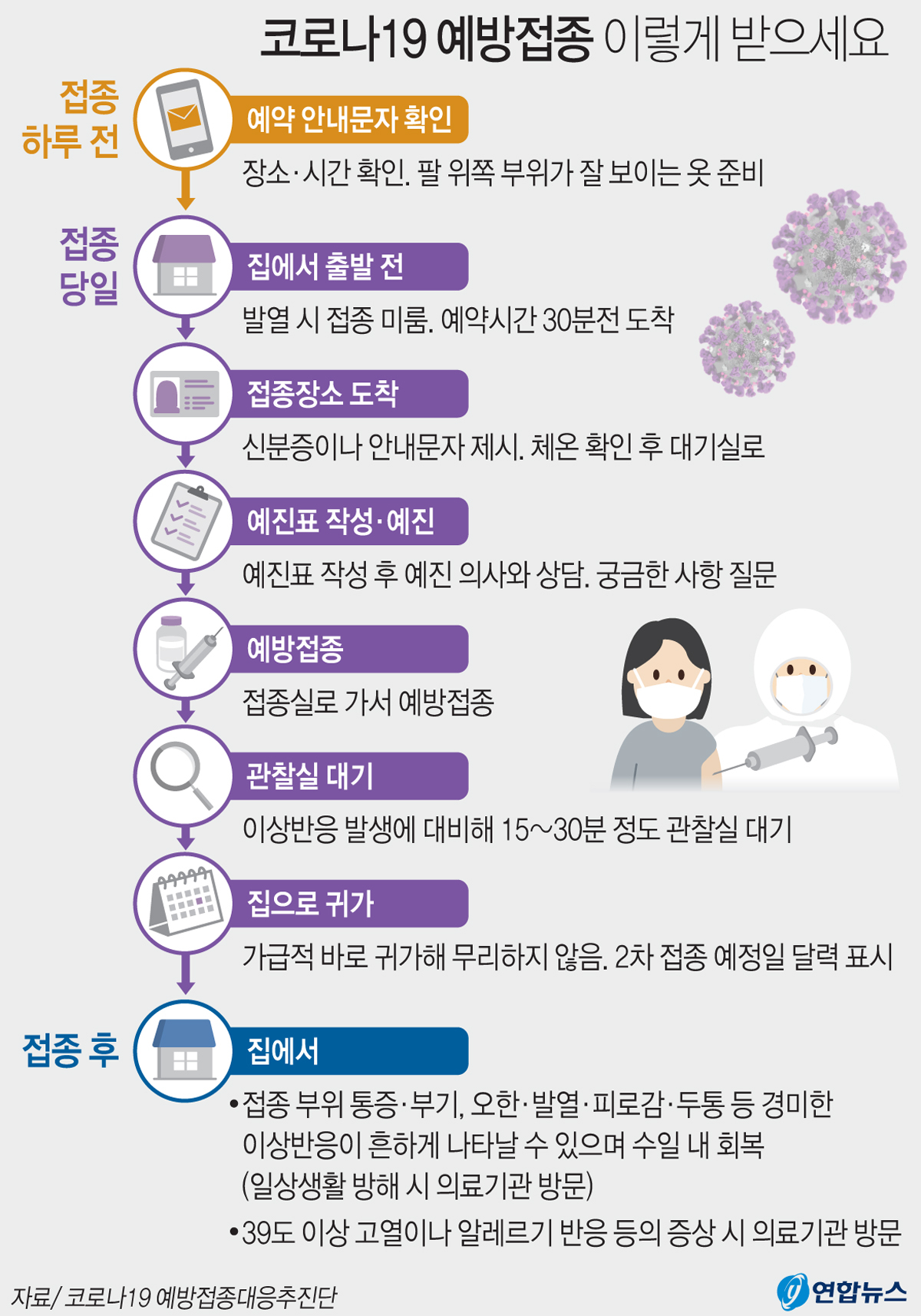
Get the COVID-19 vaccine this way. Yunhap news
Reporter Han Young-hye [email protected]
![]()
[ad_2]