
[ad_1]
Permission based on expert panel recommendation … Next week, 300 million people start getting vaccinated first.
Indian pharmaceutical company Barat Biotech’s vaccine will likely be approved for limited use

The Indian government officially approved the use of a new vaccine against coronavirus infection (Corona 19) for AstraZeneca, a multinational pharmaceutical company, the local Ministry of Information and Communication said on the 2nd.
According to the decision of the government expert panel the day before, the drug management authorities were preparing the final presentation and a senior cabinet official confirmed it first.
According to Bloomberg News and Reuters, India’s Information and Communication Minister Prakasi Javadekar said the day that “the use of the AstraZeneca vaccine has been approved.”
In India, the introduction or not of drugs such as vaccines is decided on the recommendation of a panel of experts established under the Drug Administration of India (DCGI) within the Government Agency for Drugs and Central Standards of India. (CDSCO).
Local media said that after a panel of experts made the decision to approve the use of the AstraZeneca vaccine the day before, it made recommendations to drug management authorities.
This is the first time that an emergency use of Corona 19 vaccine has been approved in India.
The countries that approved the use of the AstraZeneca vaccine before India were the United Kingdom, Argentina and El Salvador.
In India, the local pharmaceutical company Serum Institute (SII), the world’s largest vaccine company, has been conducting clinical trials of the AstraZeneca vaccine.
IBS is known to have already produced 50 million doses in preparation for approval.
The SII plans to increase production to 100 million batches per month by March.

Currently, in India, three companies, including SII, the Indian pharmaceutical company Barat Biotech, and Pfizer, have applied to the Indian authorities for the urgent use of the vaccine, and more than nine companies have started developing and producing vaccines.
In this regard, after AstraZeneca, the use of Barat Biotech’s COVID-19 vaccine is expected to be approved.
According to local media such as the Hindustan Times, a panel of government experts met again the same day and agreed to recommend that the government approve the limited emergency use of the Barat Biotech vaccine.
The Barat Biotech vaccine has been traditionally produced using an inactivated virus and is known to be effective in responding to the UK mutant Corona 19 virus.
“Corona 19 is expected to have many mutations,” said Krishna Ella Barat, president of Biotech, on 29 last month. “Our vaccine that uses an inactivated virus has a protective function against this.”
On the situation, Javadecar said that “India is probably the only country with more than four vaccines available.”
According to the approval, India plans to start vaccinating from next week.
The number of priority vaccination targets set by the Indian government is about 300 million people, including medical personnel, police, soldiers, and people aged 50 and over.
Health and Family Welfare Minister Harsi Bardan said that day, “The vaccine will be distributed free of charge to 30 million people, including medical personnel and workers at the quarantine front.”
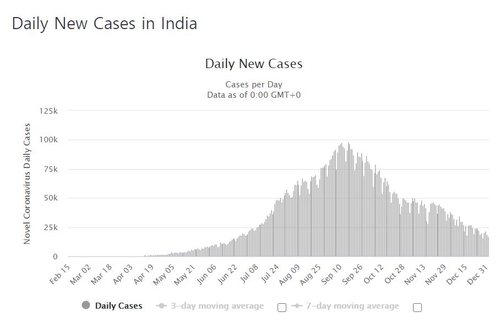
On the other hand, the cumulative number of confirmed corona 19 cases in India was 13,005,788 (according to the Ministry of Health and Family Welfare) that day, an increase of 19,000 over the previous day.
The number of new confirmed cases per day, which reached 100,000 in September, has recently dropped to around 20,000.
/ yunhap news