
[ad_1]
Free vaccination for 30 million people, including medical staff … Promote the supply of 300 million people by July
Trying to escape the stigma of ‘second place in the world’ as a fulcrum in the production capacity of the global vaccine supplier ‘Hub’

India, with a population of 1.38 billion, is entering ‘Daejangjeong’ vaccination against the new coronavirus infection (Corona 19).
The Indian government recently finalized the approval process for the emergency use of the Corona 19 vaccine developed by the pharmaceutical company AstraZeneca and the University of Oxford in the UK.
Although several countries have already approved the use of Pfizer, Moder or vaccines and have started vaccination, India approved the use of the vaccine for the first time.
Given that India is the world’s largest exporter of generic drugs and a key pharmaceutical supplier responsible for approximately 60% of global vaccine production, approval is expected to establish itself as a ‘lynchpin’ in the global supply of vaccines.
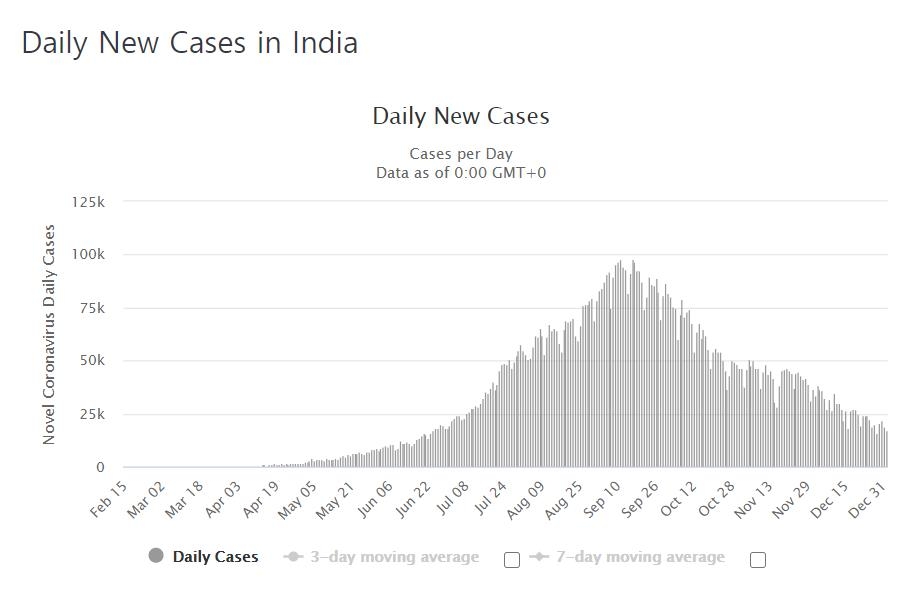
India, which has the second highest cumulative number of confirmed cases in the world (13.0578, according to the Ministry of Health, India) has been highly anticipated for the distribution of vaccines as it is one of the countries that suffered the most damage from the Corona bud 19.
In particular, the AstraZeneca vaccine has been selected as the Corona 19 “priority 0” vaccine to be introduced by the Indian government.
This is because the Serum Institute (SII), a national pharmaceutical company and the largest vaccine company in the world, participated in clinical trials and handled production through an Indian factory.
This is because the AstraZeneca vaccine is more suitable than the Pfizer, Moder or vaccine which is expensive and requires cryogenic distribution, considering the delivery situation where there are many poor people and the medical infrastructure such as the cold chain (chain of cold) is weak.
In fact, AstraZeneca vaccine can be stored under refrigerated conditions (2-8 for) for at least 6 months, but Pfizer and Modena vaccines must be distributed at cryogenic temperatures below -70 ℃ and below -20 ℃, respectively. .

IBS has already produced 50 million doses (1 dose = 1 dose) in factories in India in preparation for emergency approval.
The SII plans to increase production to 100 million doses per month by March.
Indian authorities plan to start vaccinating around 10 million health and medical workers and 20 million police, military and municipal employees starting next week.
Once vaccinations for medical personnel are completed, the number of vaccination targets increases to those 50 years and older and complications to less than 50 years.
Approximately 300 million people are eligible for these pre-vaccinations, and the government of India plans to complete the vaccinations by July.
Indian Health and Family Welfare Minister Harsi Bardan said on the 2nd that “the vaccine will be distributed free of charge to 30 million people, including medical personnel and workers at the quarantine front.”
Currently, more than 9 pharmaceutical companies, including IBS, have started developing and producing a COVID-19 vaccine in India.
In addition to IBS, Pfizer and the Indian pharmaceutical company Barat Bio have also applied to the authorities for the emergency use of the Corona 19 vaccine.
Consequently, other vaccines will be obtained one after another after AstraZeneca.
Minister Bardan said last month: “In the next 6 to 7 months, we will have the capacity to vaccinate 300 million people in India.”

Regarding vaccine distribution infrastructure, as of the beginning of last month, there are 8,5,634 refrigeration and freezing facilities for vaccine storage in 28,000,947 cold chain bases (low temperature distribution networks) in India, local media reported.
There are 50,000 cold chain technicians in 700 refrigerated trucks.
However, experts noted that in order to smoothly distribute a large number of vaccines, the infrastructure needs to be further expanded.
Consequently, it is known that the federal government of India is sending additional cold chain related facilities such as large refrigerator rooms, access coolers and freezers to each state in preparation for the full supply of vaccines.
However, India’s weather, which starts to rise above 30 ℃ in March, frequent power outages, and a lack of manpower for vaccination and follow-up management are expected to hamper vaccine supply.
Meanwhile, the spread of Corona 19 in India, which had a number of confirmed cases approaching 100,000 a day in September, has slowed significantly recently.
The cumulative number of confirmed cases on this day only increased by 19.79 from the previous day.
/ yunhap news