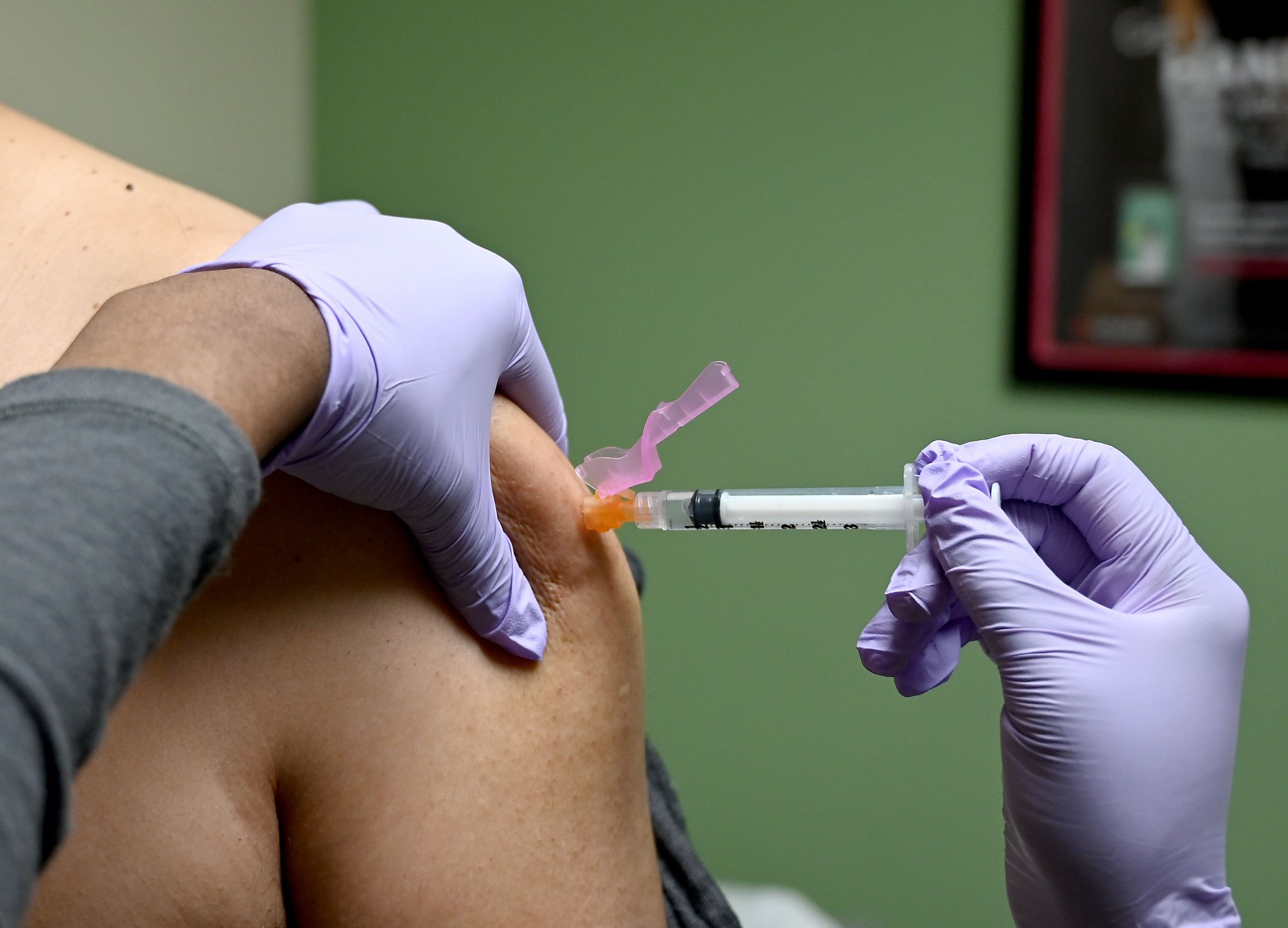
Kaiser Permanente has participated in a Phase 3 clinical trial to test a coronavirus vaccine candidate currently in production by Pfizer and BioNTech, and will visit 1,400 adults aged 18-85 in California and Oregon to sign up to participate in the study, a press release from the medical organization states. The goal of the trial is to enroll 30,000 patients in 120 sites around the world to participate.
According to Kaiser’s research database, some of these vaccine candidates will be administered in Northern California at Santa Clara Medical Center and Sacramento Medical Center. Registration for the trial has not started yet.
The study will be a double-blind, where half of the enrolled patients will receive the vaccine candidate called BNT162b2, and the other half will receive a placebo. Neither the patients nor the researchers will know what dose participants received.
“Kaiser Permanente is extremely well positioned to address this public health emergency,” said Dr. Nicola Klein, director of the Kaiser Permanent Vaccine Study Center in Oakland in a statement. “We have been a leader in vaccine research for over 30 years and have participated in clinical trials for almost every vaccine licensed in the United States. We know we can make a significant contribution to helping determine if this vaccine is effective in preventing it. of COVID -19 disease. “
Research into the vaccine will be conducted on three Kaiser research sites on the West Coast: the Oakland Research Department, the Pasadena Research and Evaluation Department and the Kaiser Permanent Center for Health Research in Portland, Ore.
The world is currently without a reliable vaccine candidate that believes in protecting against SARS-CoV-2, the new coronavirus that causes the respiratory disease COVID-19. On Tuesday, Russian President Vladimir Putin announced that he had approved a coronavirus vaccine for widespread use, despite cautious concerns from the international medical community that the vaccine was not ready for use because it had not completed testing. Putin noted that his daughter was among the first to receive the vaccine.
US President Donald Trump told media later Tuesday that US health companies are introducing their vaccine candidates in Phase 3 trials, and that the federal government is concluding contracts with Johnson & Johnson, Sante Fe, Moderna and others to produce hundreds of millions of doses ready for deployment as soon as possible if it is approved by the Food and Drug Administration.
When he told the media, “[There’s] enormous premise in each of them. “
MORE CORONAVIRUS COVERAGE:
Sign up here for ‘The Daily’ newsletter for the latest on coronavirus.
“Fear of a neck injury can be worse than no mask at all,” said researchers
—SF Italian restaurant that transformed into general store calls it closing
—We hope you remember our brewery ‘: Armstrong Brewing of South San Francisco closes permanently
—Bryan Cranston inspired by Tom Hanks to open COVID-19 diagnosis, donate plasma
—SF will spend more than $ 446 million on COVID response, but will need more funding after June 2021
Alyssa Pereira is a culture editor at SFGate. Email: [email protected] | Twitter: @alyspereira
Recent Comments