[ad_1]

A needle is filled with a vial of the Covid-19 vaccine at the Royal Victoria Infirmary in Newcastle, UK, on Tuesday, December 8, 2020. Vaccine injections, created by Pfizer Inc. and BioNTech SE, have been given available to 50 hospitals across the country, before being distributed to vaccination centers run by physicians who will administer the injections.
Photographer: Owen Humphreys / PA Wire
Photographer: Owen Humphreys / PA Wire
Every morning we deliver world news that you want to keep track of before starting your day. Click here to subscribe to the Bloomberg newsletter.
British pharmaceutical companyThe British government is ready to approve a new vaccine against coronavirus infection (COVID19) jointly developed by AstraZeneca and the University of Oxford. It is expected to be a powerful new means of countering the new crown in a situation where there is concern about the spread of infection.
British drug regulators and supervisors may approve the use this week, officials said on condition of anonymity due to the nondisclosure of the talks. Pascal Sorio, AstraZeneca’s chief executive, and British health officials have previously said they await approval later this year.
Approval of the new vaccine is expected to be about three weeks after the UK started vaccination in Western countries. The vaccine from Pfizer in the US and Biontech in Germany has been given to more than 600,000 people in the UK, but the number of infected people continues to rise amid growing anxiety over variants said to be more infectious.
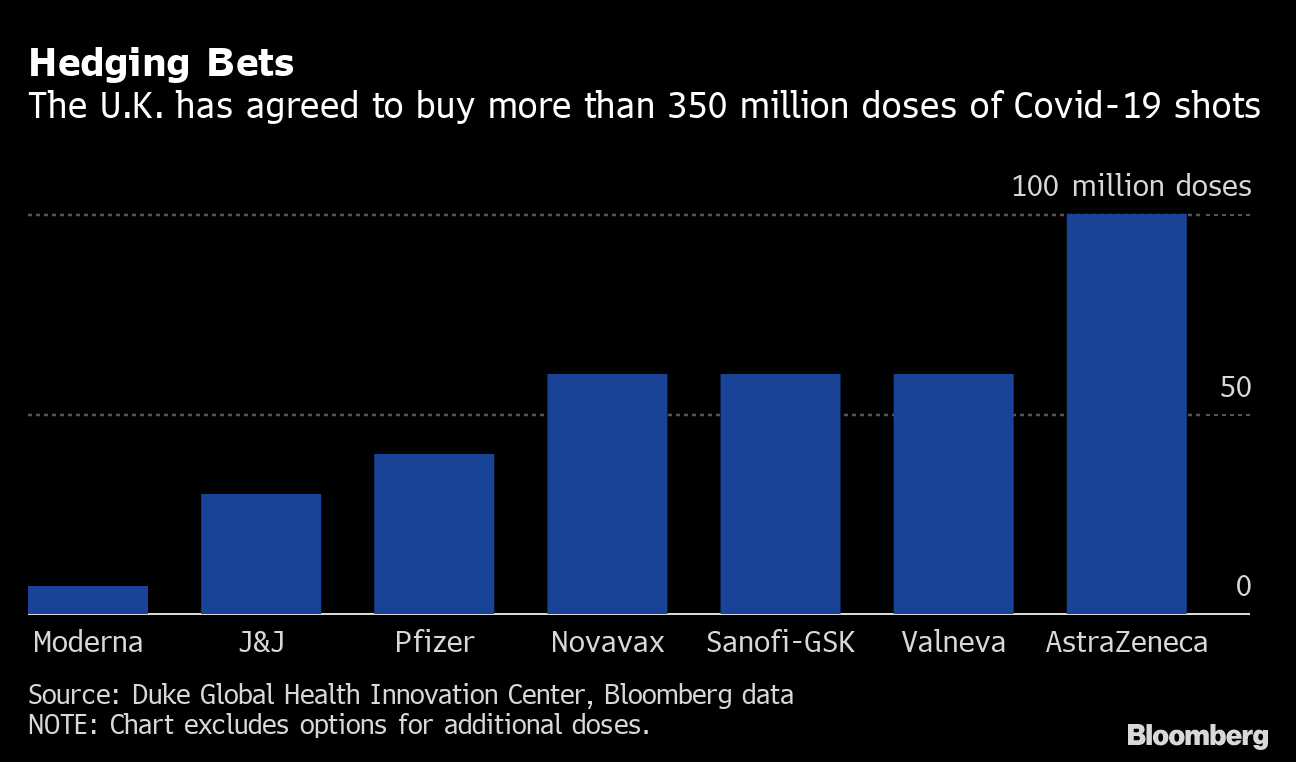
Hedging bets
The UK has agreed to buy more than 350 million doses of Covid-19 injections
Source: Duke Global Health Innovation Center, Bloomberg data
British newspaperAccording to Mail on Sunday, officials expect that by March 20 million people at increased risk will be vaccinated if the AstraZeneca and Oxford University vaccines are approved.
UK shutdown, mitigation end of February, with AstraZeneca vaccine
Sorio CEO is an English Sunday newspaperIn an interview with the Sunday Times, new data will show that the efficacy of AstraZeneca’s vaccine is comparable to the 95% reported by rival companies.
According to the British Telegraph, mass inoculation in stadiums and conference rooms is expected to begin in the second week of January. Meanwhile, scientists have mentioned that schools may need to be closed to slow the spread of the variant, and government officials will meet on the 28th.
Original title:
UK prepares to phase out AstraZeneca vaccine as need for vaccines grows (抜 粋)