[ad_1]
Side effects of vaccination with the new coronavirus infection (COVID19) include fever, cold, headache, and joint pain, and if side effects do occur, some doctors and nurses may not be able to work.
If the US Food and Drug Administration (FDA) issues an Emergency Use Permit (US),Pfizer andThe Modelna vaccine may begin shipping in the coming weeks, and medical institutions are preparing to vacate key hospital personnel.
This week, the US Center for Disease Control (CDC) Advisory Board will prioritize vaccinations for healthcare workers and residents of long-term care facilities.Recommended. According to data from the COVID Tracking Project, more than 100,000 people in the United States were hospitalized for the new corona infection as of day 2, causing serious problems for hospital staff due to vaccination of medical personnel. There is also fear.
Medical personnel should leave the workplace for vaccination and go to the vaccination clinic. In the event of side effects, key personnel may be absent from work for several days. To overcome this situation, some hospitals change staff working hours, and some medical institutions seek to evict staff before the end of work shifts and some days off.
Serve as an emergency response executive at Massachusetts General HospitalPaul Bidinger says it’s hard to see the future without enough data from the large, late-stage clinical trials of Pfizer and Moderna.
Although the two companies have not yet fully released the clinical test results, the test results so far disclosed in the recent press release show a certain safety profile.
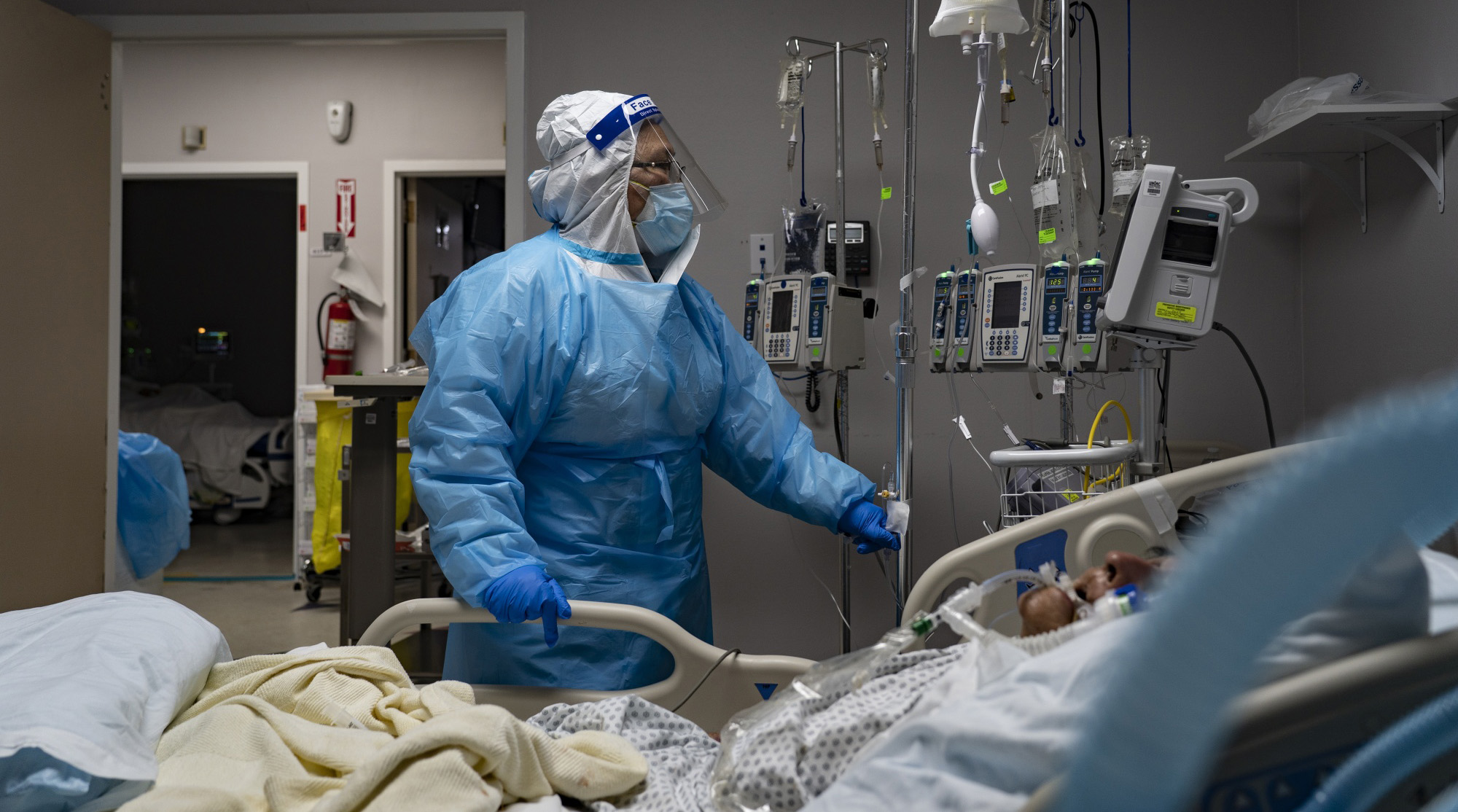
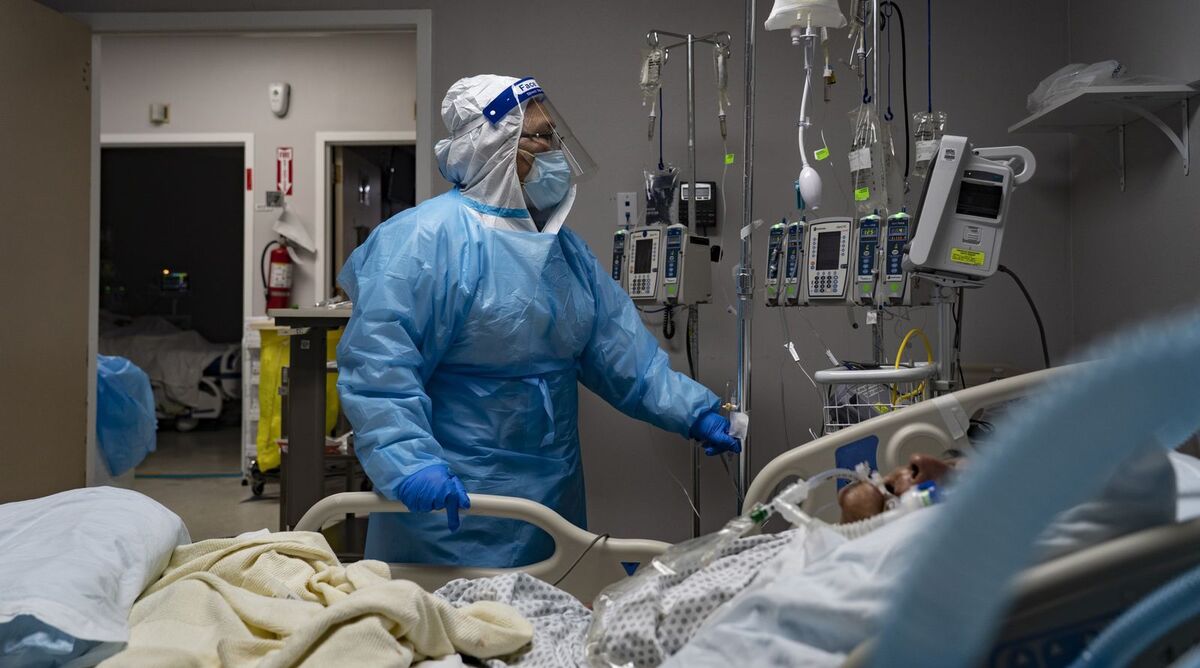
Treatment of medical staff in the new intensive treatment room for Corona patients at a hospital in Houston, Texas (November 8)
Photographer: Go Nakamura / Bloomberg
Pfizer and its partner, Biontech, GermanyOn November 18, it was reported that no significant safety concerns were observed in the late clinical trials. Of the participants who received two vaccines, 3.8% experienced discomfort and 2% experienced headaches. Participants who were relatively older had few reports of adverse events and they were mild. Previous tests showed mild to moderate feverconfirmed.
Meanwhile, Moderna said Nov. 16 that no significant safety concerns were identified in the late-stage clinical trials.Ad. Mild to moderate side effects included malaise (9.7%), muscle and joint pain (5.2%), headache (4.5%), and injection site pain (2.7%) . Side effects were more common after the second of the two inoculations.
“We are very relieved to have no unexpected symptoms. Side effects are common with other vaccines we use,” said Buddy Creech, director of the Vaccine Research Program at Vanderbild University. Is. “” Not everyone has a fever or a cold, “he said.
Original title:
Vaccine side effects put healthcare workers at risk amid the surge (抜 粋)