[ad_1]
MeterPfizer and GermanyBiontech has requested the approval of the European Union (EU) authorities for the new jointly developed coronavirus vaccine. Approval is expected to be granted by the end of the year.
The European Pharmaceutical Agency (EMA) said it could announce its views in a few weeks a day, and said it would meet no later than 29 this month. Both companies submitted a formal approval request on November 30. European authorities had started a “sequential review” on October 6 to examine data on the vaccine from time to time.
The Modern American competition also sought approval from the US and European authorities on the 30th on the corona vaccine. Governments want to start vaccination as soon as possible, and in the UK there are special rules that allow national pharmaceutical authorities to make their own. decisions without waiting for the EMA’s decision.It was activated.
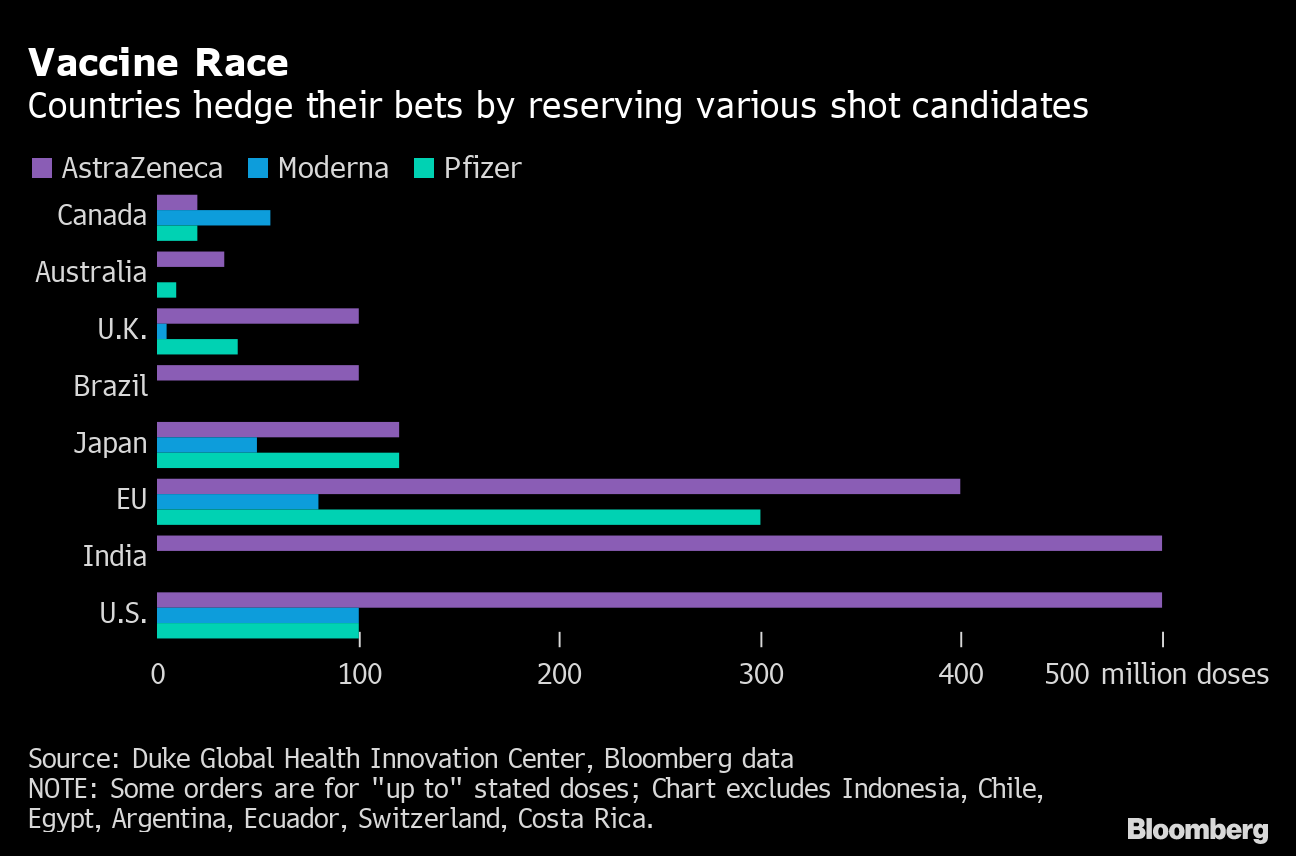
Vaccine race
Countries hedge their bets by reserving multiple shooting candidates
Source: Duke Global Health Innovation Center, Bloomberg data

Biontech CFO Zirk Petting told a press conference that the first shipment could begin “in a few hours” after approval from authorities. On the German stock market, the company’s shares temporarily rose about 11%.
If the EMA concludes that the benefits of the vaccine outweigh the risks, conditional marketing approval (CMA) will be granted and distribution will be available in Europe later this year, Physer and Biontech said in a statement. .. The EMA explained that this evaluation work would be done “throughout the Christmas period”.
The two companies have also started filing applications with authorities in other countries such as Australia, Canada and Japan. It has agreed to supply the EU with 200 million doses and give it the option to add another 100 million doses.
Original title:Pfizer and BioNTech seek EU authorization for Covid-19 vaccine (2) (抜 粋)
(We will update the fifth paragraph with the content of the announcements of Physer and Biontech, the reaction of EMA, etc.)