[ad_1]

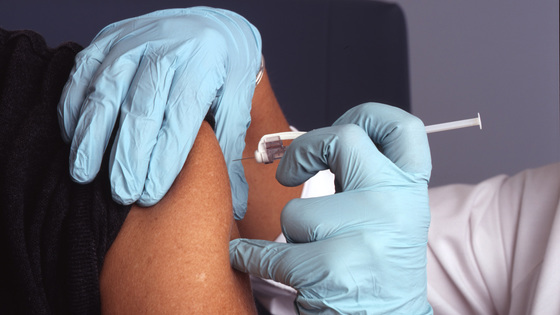
Moderna, a biotechnology company developing the new corona virus vaccine “mRNA-1273,” has announced the final results of a clinical trial involving approximately 30,000 subjects. According to him, the effectiveness of the vaccine was 94.1% and the effectiveness against aggravation was 100%. In response, Moderna has applied for an emergency license from the United States Food and Drug Administration (FDA).
Moderna Announces Primary Efficacy Analysis in COVE Phase 3 Study for its COVID-19 Candidate Vaccine and Filing Today with the US FDA for Emergency Use Authorization | Moderna, Inc.
https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-primary-efficacy-analysis-phase-3-cove-study
‘Absolutely Remarkable’: No one who received Moderna’s vaccine in the trial developed severe COVID-19 | Science | AAAS
https://www.sciencemag.org/news/2020/11/absolutely-remarkable-no-one-who-got-modernas-vaccine-trial-developed-severe-covid-19
MRNA-1273 is different from conventional vaccines that detoxify or attenuate the virus itself and purify it.MRNATo acquire immunity usingmRNA vaccineIt features faster development, a simplified manufacturing process, and higher manufacturing cost performance than before.
In fact, mRNA-1273 was the world’s first human clinical trial as a novel coronavirus vaccine on March 16, 2020, just two months after obtaining the novel coronavirus genome sequence.
The world’s first human clinical trial of a new coronavirus vaccine has begun – GIGAZINE
Moderna’s mRNA-1273 was developed as part of the “Operation Warp Speed Program” to drive the development project for the new coronavirus vaccine and, in mid-November 2020, is the final stage of Phase III clinical trials. (Phase 3). ), And it was announced that the effectiveness of 94.5% was confirmed.
Moderna Announces New Corona Vaccine Under Development Has Achieved “94.5% Preventive Effect” In Clinical Trials – GIGAZINE
According to the final report, mRNA-1273 worked to the same extent in all different groups, races, and genders. “MRNA-1273 is very effective against mild and severe symptoms and is most effective when the vaccine is given to people at increased risk of new coronavirus infections,” said Stephen Bansel, CEO of Moderna. In other words, priority should be given to healthcare workers, the elderly, diabetic patients, obesity and people with heart disease. “
Moderna expects to provide the US government with 20 million doses of vaccines by the end of the year, and said it plans to provide 50 million doses, taking into account other countries that have pre-purchased contracts with the United States.
According to Vansel, the cost of vaccination in developed countries ranges from $ 32 (around 3,300 yen) to $ 37 (around 3,800 yen) each time. However, Moderna is affiliated with the non-profit organization “COVID-19 Vaccines Global Access Facility” that advocates for vaccine equality, and it appears that it may be cheaper in low-income countries.
Copy the title and URL of this article.
[ad_2]