[ad_1]
AstraZeneca and the University of Oxford have admitted that there was an error in the manufacturing process of the new coronavirus vaccine, which was developed jointly, raising questions about the results of the vaccine.
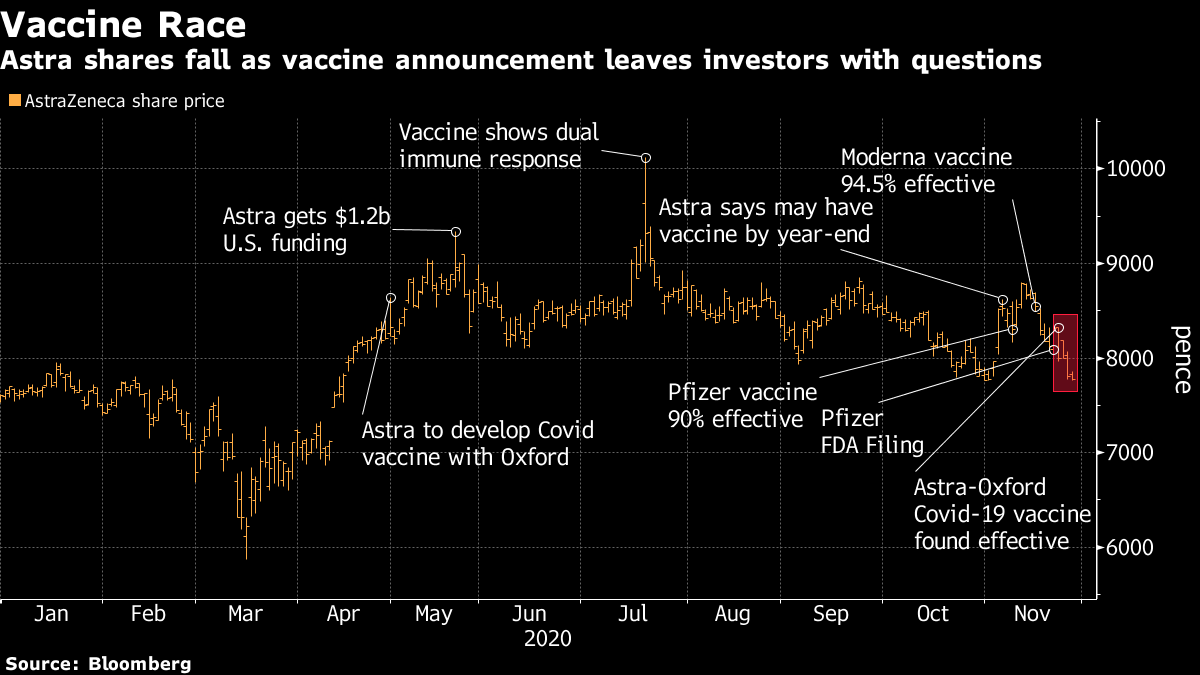
AstraZeneca and the University of Oxford announced this week that the vaccine had an average 70% chance of being effective in a phase 3 clinical trial. Some say US authorities will waive the license due to a lack of published data.
AstraZeneca and the University of Oxford say that half the dose followed by a full dose was 90% effective, and two doses were 62% .However, the day after the announcement, the person in charge of the US administration vaccines “Operation Warp Speed” said that the high probability of effectiveness was demonstrated in a trial in relatively young subjects.He pointed.
Oxford University revealed that due to variations in the manufacturing process, half the dose was administered rather than a single dose at the end of the study. When this was discovered, it was agreed with the authorities to continue the study with two different doses.
“The method of measuring the concentration of vaccines has already been established, so it is now certain that all vaccines are equivalent,” explained the university.
“The most likely explanation for the interim analysis showing different efficacy was by chance or by subject composition,” said Sam Fazeli, an analyst at Bloomberg Intelligence. In any case, current data. If approved on the basis of, people will be vaccinated with vaccines of unknown true efficacy. “
An AstraZeneca spokesperson explained that the trials were conducted to the “highest standards” and additional analyzes were performed to ensure that the efficacy figures were accurate.
Original title:
Astra faces more questions about vaccines after manufacturing error (抜 粋)