
[ad_1]
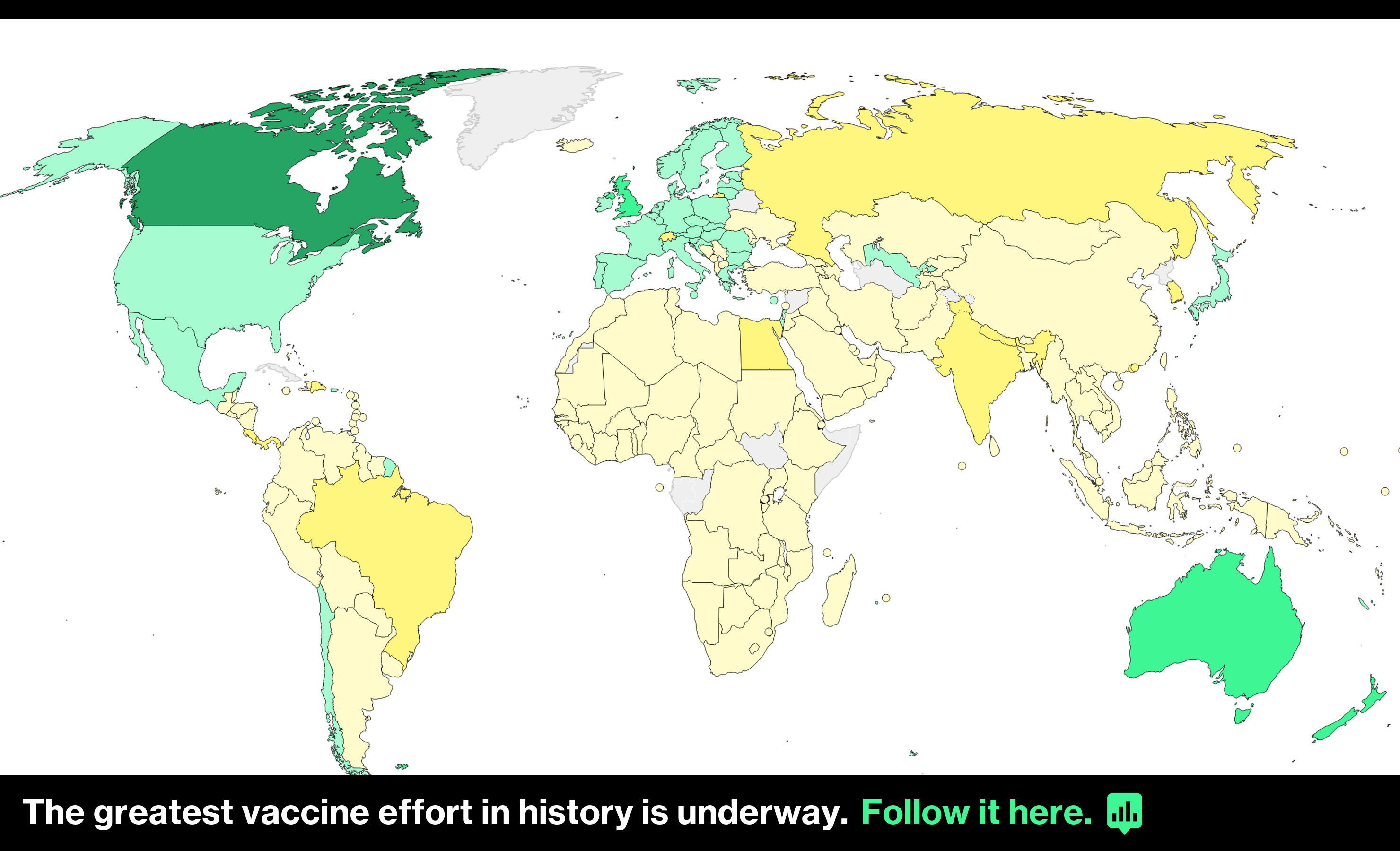
The European Medicines Agency (EMA) announced on the 18thIt was concluded that the new coronavirus vaccine manufactured by AstraZeneca in the UK can be used safely. Despite safety uncertainties, stalled European Union (EU) vaccination has advanced.
EMA officials told a news conference that the benefits of using Astra vaccines were “safe and effective” and clearly outweighed the risks. Following reports of thrombosis after vaccination, the vaccine was used in European countries.The movement to suspend use was widespread.
Following the EMA’s findings, Germany, France and Italy, as well as Slovenia and Luxembourg will resume the use of Astra vaccines. Other countries are likely to follow suit, but Sweden has withheld its decision to continue with its own investigation.
EMA recommends adding a warning to the vaccine to ensure that the information is well known. Although the number of cases of blood clots is extremely limited and not related to vaccination, experts say the causal relationship cannot be “conclusively” ruled out.
The UK Medicines and Healthcare Products Regulatory Agency (MHRA) shared a similar opinion on the safety of Astra vaccines on the same day. However, a rare case of platelet depletion and blood clots in the brain after vaccination was found in five men aged 19 to 59, one of whom died and is being investigated for its relevance with experts.
“The Oxford vaccine (AstraZeneca) is safe. The Pfizer vaccine is also safe. The unsafe thing is that it affects the new Crown,” British Prime Minister Johnson told a news conference. It was revealed that it would be inoculated.
AstraZeneca has been determined by the EU and UK pharmaceutical authorities and has stated that it will work closely with health authorities to ensure proper use of the vaccine.
Original title:The EU regulator considers Astra to be safe, but recommends a warning label (2) (抜 粋)
Europe restarts Astra vaccines after safety approval (1) (抜 粋)
UK regulator says benefits of Astra Covid shot outweigh risks (1) (抜 粋)
UK’s Johnson says he’ll get Astra Shot on Friday (抜 粋)
(We will update adding moves to resume use in major EU countries.)