[ad_1]
The American biotechnology company Cellex has announced that it has completed the development of a new rapid test, which will be used to detect the presence of the coronavirus with a DIY kit, without the need to undergo a swab to be analyzed in the laboratory. Interpretation of the result can be done through an application, developed in collaboration with Gauss, an IT company specialized in artificial intelligence systems. The two companies are confident that they will be able to bring their test to market shortly, through an emergency approval from the Food and Drug Administration (FDA), the US federal agency that deals with drug and device safety. doctors.
PCR test
As we have learned in recent months, the most common test to detect the presence of the coronavirus consists of subjecting a sample of mucus and saliva, using a swab that is then analyzed in the laboratory for the genetic material of the virus (PCR technique) . The testing process requires expensive machinery and several hours to complete, which does not always allow for the coronavirus to be detected in a timely manner in people who may be infected.
Antigenic test
The test developed by Cellex works differently: Instead of looking for the genetic material of the coronavirus, it looks for particular proteins typical of the virus. It is called “antigenic test” because it actually detects the presence of certain foreign substances in our body (the word “antigens” defines the foreign substances present in our body that cause a reaction, with the consequent production of antibodies).
Antigen tests can provide results in minutes and are less expensive than PCR tests. Therefore, they are more suitable to be carried out in a large number of people, but they have some reliability problems. Simplifying: if an antigen test is positive, the result is very reliable, if it is negative, there could be problems with false negatives: the person being tested is actually positive for the virus and the test did not detect it. For this reason, negative antigen tests often carry out a PCR test, more reliable in the event of a negative result, to determine whether or not the person being tested is infected.
Antigens and antibodies
Antigen testing should not be confused with serologic testing, which instead detects the presence of antibodies, but not necessarily an ongoing viral infection. Antigen and PCR tests are like a photograph: they tell you whether or not you have the coronavirus at the time. Serological tests, on the other hand, indicate whether or not one has come into contact with the virus, in recent times or more ago.
The new rapid test
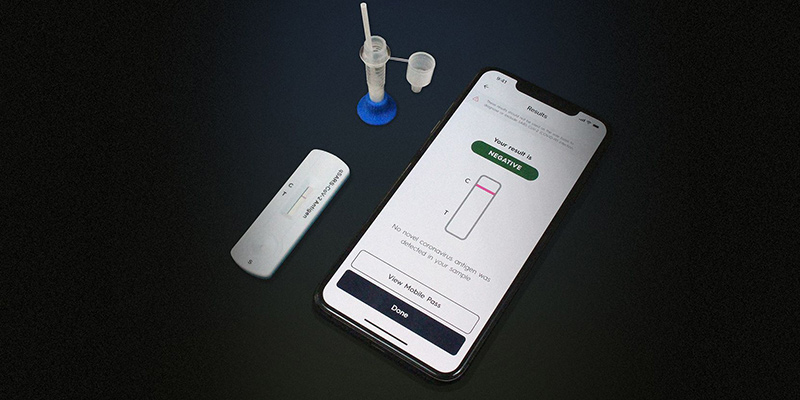
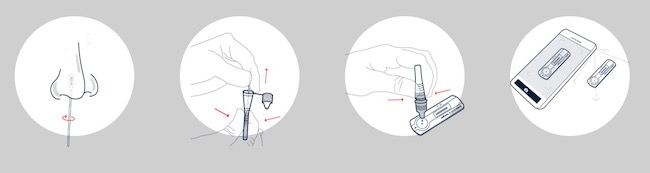
The rapid test developed by Cellex falls into the category of antigen testing. In the kit there is a swab, smaller than those used for PCR tests, and which must be passed through the interior of the nostrils, without reaching the deepest parts of the nasal septum. The swab should be placed in a small plastic tube with a reagent, which after a few minutes should be poured into a tester (a small plastic box).
(Cellex / Gauss)
In about 15 minutes, colored lines appear on the tester that must be photographed with the application developed by Gauss. The application analyzes the image and within seconds provides instructions to interpret the result. The idea is to take advantage of the application’s image recognition capabilities to avoid incorrect test readings, especially if the tester lines are not very visible or give a seemingly contradictory result.
Cellex is not the only company that has developed a rapid antigen test. Sona Nanotech of Canada has built a similar system, without application, and has applied for FDA approval. At the moment, the moment of approval is not clear, because much depends on the scientific evidence that companies provide to demonstrate the effectiveness of their systems.
In recent months, numerous governments and health institutions have promoted the development of rapid tests to detect the presence of the coronavirus, with the aim of reducing analysis times and the strong pressure on laboratories, which have been analyzing samples continuously for months. A faster system could allow better control of population groups at higher risk, as well as detect new outbreaks before they cause a large number of new infections.
However, not everyone is convinced that antigen testing can be a good solution, given the risk of numerous false negatives. However, according to some observers, suspected cases could only be tested for antigen, compensating with PCR only in the case of negative test results.
[ad_2]