[ad_1]
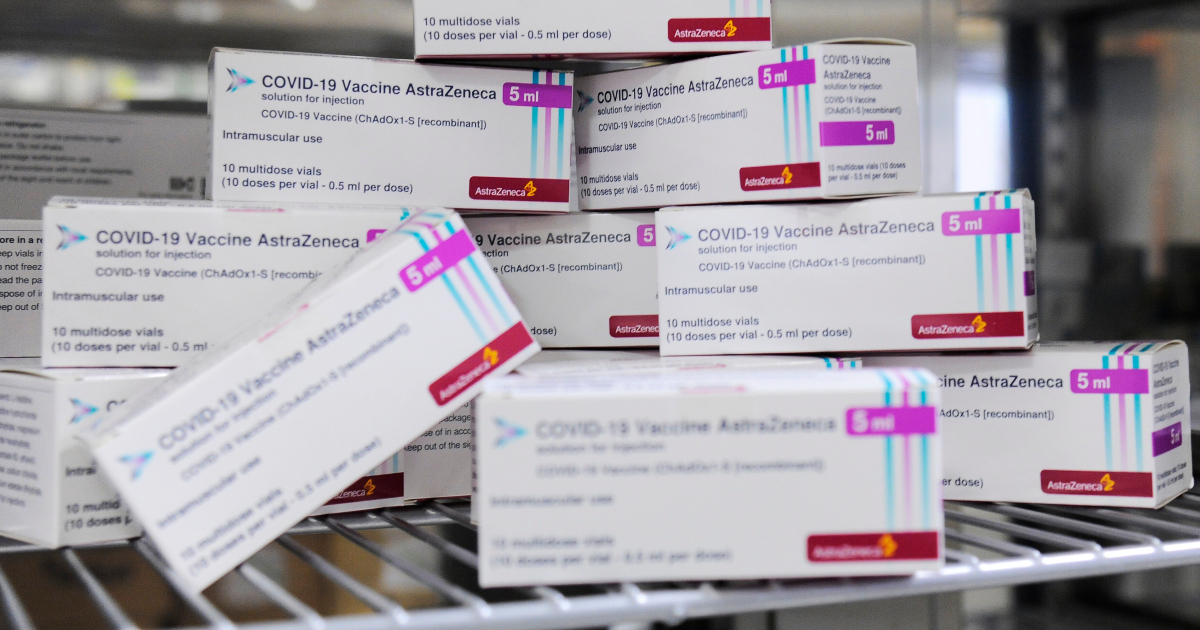
At the Catalent plant, ad Anagni, there are “29 million doses of Astrazeneca vaccine ready to go for UK“. The revelation of The impression, relaunched by Bloomberg, yes confirmed Risks of causing a new upheaval in relations between the pharmaceutical company and the European Union, which have already deteriorated after the breach of deliveries expected in the first six months of the year. According to reports from the Turin newspaper, the Italian authorities have discovered the existence of this stock of vials after an investigation carried out in reporting of the European Comission. A passage confirmed in these hours by sources quoted byAnsa: between Saturday and Sunday i Nas of the Carabinieri inspected the plant by mandate of the Ministry of Health. The batches that Brussels asked the Italian Prime Minister to verify resulted in destination Belgium (where the last step in the vaccine supply chain is). Asked about the matter, the Executive Vice President of the EU Commission, Valdis Dombrovskis, stated that “it depends on the company to clarify what intentions do you have. For our part, we can only say that AstraZeneca is far from respecting the commitments provided in the contract“Since they should administer 30 million doses in the first quarter and today” they are not nowhere near that figure“.
History, writes The impression, would emerge early march, when the commissioner Thierry breton i visited him establishment say leida, in the Netherlands, managed by Halix, which together with the Belgian company Seneffe produces the drug on the EU territory on behalf of Astrazeneca, while the filling is carried out in Anagni. The point is, write The impression, the Dutch plant has not yet obtained authorization by the European Medicines Agency. The doses it produces, therefore, cannot be delivered to EU countries, so much so that “it is very likely that in a first phase they were Sent in the UK. “Then, with the introduction in late January of the export control mechanism on the part of the EU Commission, everything would have stopped.
Meanwhile, however, the production chain – started in September and able to turn out 5-6 million doses per month – would never stop. The result is that the vials have accumulated in warehouses: the first report sent to Brussels – writes the Turin newspaper – says that in refrigerators in the warehouses of the Lazio site there are 29 million doses of the vaccine. EU sources let it be known that not all were produced in Leiden, but are still ready-to-use vials. be injected and destined for several non-EU countries (in addition to Great Britain, also those that are part of the plan Covax). Why has the factory that produced them not yet been authorized by the EMA? The impression reports that the EU institutions suspect that the delay of the company in providing the necessary data and documents is due to a tactic to be able to guarantee the United Kingdom a Bus Lane in the delivery of doses. History, therefore, would be at the center of the dispute between London and Brussels that has been going on for weeks. 29 million doses is a huge stock, equal to double of which the Anglo-Swedish house has so far delivered to the European Union, therefore capable of make up for delays accumulated in the vaccination campaign. For the British, on the other hand, these are essential vials to maintain the speed achieved so far in the injections and to guarantee everyone the second dose of vaccine.
The first reaction to the news, very harsh, comes from Manfred weber, President of the EPP (popular) group in the European Parliament: “Necessary and urgent explanations are needed! AstraZeneca is storing tens of millions of doses without respecting the European contract, ”he wrote on Twitter, relaunching an article on French radio. Europe1. This is unacceptable. The urgency is enormous. We should categorically refuse any Astra Zeneca export produced in Europe“In the background there is in fact the decision of the EU Commission to strengthen even more the export control mechanism, introducing the criteria of “reciprocityAnd “proportionality” with the recipient countries. A system today explained in detail at a press conference and designed precisely to prevent doses of Covid vaccines produced on European soil ending up in the United Kingdom without receiving anything in return.
Since their introduction they have been welcomed in total 380 export requests toward 33 countries for a total of approx. 43 million doses. Not a single export application was accepted, reports the Commission. The main export destinations include the United Kingdom (with approximately 10.9 million doses), Canada (6.6 million), Japan (5.4 million), Mexico (4.4 million), Saudi Arabia (1.5 million), Singapore (1.5 million), Chile (1.5 million), Hong Kong (1.3 million), Korea (1.0 million) and Australia (1.0 million). The problem, Dombrovskis recalled, is that “the European Union has exported to UK 10.9 million doses “of vaccines since the end of February, but from the UK to the EU” has reached zero. ” Ursula Von der Leyen added that “roads should two-way running. Therefore, the European Commission will introduce the principles of reciprocity and proportionality into the existing Union authorization mechanism. We need to ensure timely and sufficient deliveries of vaccines to EU citizens. Every day counts. “The full dossier will also be discussed by the leaders of the 27 member states in Council of the EU scheduled for March 25 and 26.
[ad_2]