[ad_1]
Many people claim that Covid-19 vaccine it was produced too quickly and studied too little, undermining its security. So this is not the case.
The extraordinary situation made it possible to accelerate the entire process

The Pfizer / BioNTech Covid Vaccine
Under normal conditions, the development of a new vaccine can take between 10 and 15 years from the beginning of the research to its launch. This great time is necessary to complete a series of steps necessary for the new vaccine to be approved and administered on a large scale.
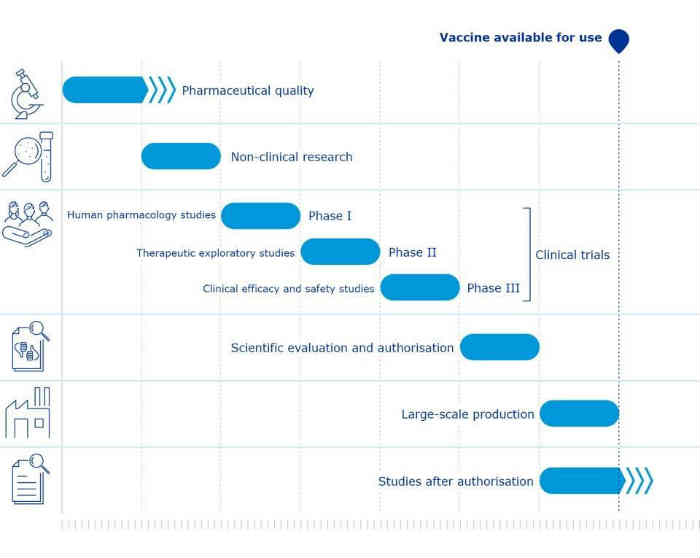
As to COVID-19 pandemic also in this case I various clinical trials they were carried out with the same rigor and with the same duration. What has changed is that Funding, bureaucracy, search for facilities and volunteers, and final evaluation were sped up..
To counteract this pandemic, the different companies immediately allocated urgent funds, thus shortening the entire process in 1-3 years, which in its initial stages usually took a long time precisely. seek funding to support the research itself. Furthermore, hospitals and universities immediately accepted to host the trials and several volunteers were immediately found meeting (if not more) the standards, allowing save a total of about 2 years.
Regarding the technology used, the famous mRNA – This method was already known to researchers from previous studies on SARS and MERS. This factor contributed considerably to shortening the time, allowing the researchers shorten this process by about 5 years. Also, the fact that this technology should not be studied in cell cultures, as it is an unnecessary phase for Covid-19, has shortened the time by one year.
Phases of clinical trials
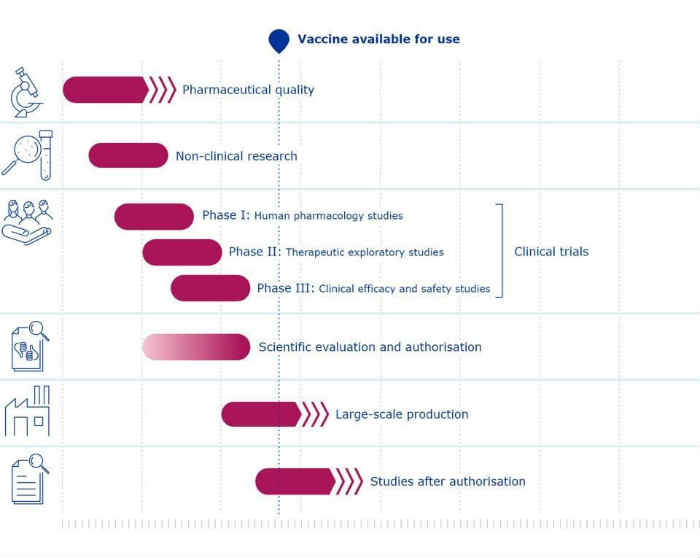
With reference to the phases of the clinical trial, these it has always been 3 of 3 to 6 months each:
- Phase I study: clinical trial involving the use of a drug for the first time in humans, with the aim of defining its toxicity. The purpose is identify the maximum safe dose with which this drug can be administered to patients without excessive risk of toxicity
- Phase II study: aims to evaluate the activity of the drug or drug combination against a specific disease and its toxicity at the previously selected dose. Once the results of the phase I study have established that a patient can receive a dose of the drug without undue risk, an attempt is made define its activity against the disease, based on the almost always true assumption that if a drug keeps the disease under control, it will produce a benefit for the patient.
- Phase III study: aims to evaluate the benefit for patients, comparing a group of patients treated with the treatment that was active in phase II with another group treated with the standard therapy for the disease in question, that is, the one already adopted by the scientific community as the best available
Why concern Covid-19 vaccine, these three phases were carried out practically simultaneously with each other, with the risk that a problem that arose in one phase would have caused the cancellation of the entire experiment. However, these problems did not arise and this acceleration meant a saving of 6 months.
Finally, at the end of these three phases, the competent authorities immediately evaluated the data provided by the pharmaceutical companies, dedicating many critics.
In this way, I vaccines against Covid-19 developed this year had priority and absolute priority over other drugs awaiting trial, causing the different approval committees to meet even in extraordinary sessions a few days after receiving the safety and efficacy data. This step meant that The whole process was shortened by another 3-4 years..
[ad_2]