
[ad_1]
To get permission to vaccine of AstraZeneca me Oxford we still have to wait: the cold shower comes from the deputy executive director of the European Medicines Agency, Noel wathion, that in an interview with the Belgian newspaper The newspaper called “unlikely” a green light fromMother a January. “They have not yet been implemented,” added Wathion, emphasizing that “they are necessary Other data on vaccine quality. “Britain’s regulatory body, Mhra, should authorize the drug shortly and the British hope they can begin distribution as soon as January 4th. A news that also gave hope to Europe: after the start of vaccination with Pfizer, the green light of theMother to the drug made by Modern.
The words of the EMA’s Deputy Executive Director are bad news in particular for Italy. the vaccine of AstraZeneca and the University of Oxford is developed in collaboration with the Italian company Irbm of Pomezia. “The viral vector is produced Pomezia, at the Irbm plant, filling is carried out in Anagni and the storage of doses does not need temperatures 75 below zero. It means that for us, using Pratica di Mare as a center, everything will be simpler: production, distribution, conservation, ”the Health Minister explained on Monday. Roberto Speranza. Speranza himself had underlined why the green light for the vaccine is crucial for Italy AstraZeneca me Oxford: “If he reaches the finish line immediately, they will join in the first quarter another 16 million doses, which correspond to another 8 million vaccinated people. Final result: we could already from April 1 have 13 million vaccinated and thus we would have already reached Phase One, that is, the one that allows us to have the first epidemiological impact”.
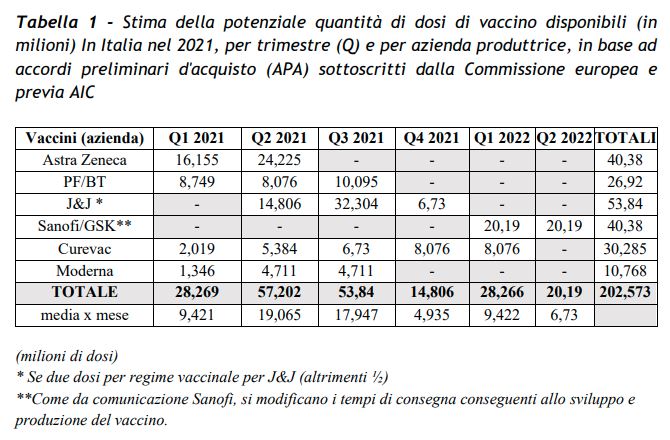
The objective of 13 million immunized people in three months it would be a risk if there were more delays in authorization. As it is written in the Government’s Strategic Plan, Italy will have 40.4 million doses AstraZeneca, 26.9 million Pfizer / Biontech, 50.8 million Johnson and Johnson, 40.4 million Sanofi / GSK, 30.9 million Curevac, 10.8 million Modern. The total figure corresponds to 13.46% of the approximately 200 billion doses purchased at the European level. But timing is also crucial: Pfizer / Biontech has already received the green light, Moderna is in the works. Others? The potential number of doses vaccine available estimated by the government in the Strategic Plan foresees, as explained by Speranza, that 16 million doses of AstraZeneca will arrive in the first quarter, then another 24 million in the second quarter (between April and June). In total they correspond to a little less than half of the total doses that the government estimates it has available in first six months of 2020 (28 million in the first quarter, 57 million in the second).
Ema Wathion’s Deputy CEO at the Belgian newspaper The newspaper He also explained that the EMA does not currently have enough information on the vaccine, as “AstraZeneca only provided data on own clinical studies“, And these” are not sufficient “to grant a marketing authorization conditioning. “We need data additional on the quality of their vaccine, ”Wathion added. Furthermore, he emphasized, AstraZeneca has not yet submitted a Formal request, what is another necessary condition to give the green light to the vaccine. In the meantime, however, the UK plans to launch the AstraZeneca-Oxford vaccine from January 4th. Why concern reason for which it would have been authorized there “,we are in the dark, because we don’t know what data AstraZeneca has submitted to the UK authorities, ”said Wathion. But it could also be that the UK agency is granting provisional authorization“, Which would only allow the distribution of certain lots of the vaccine. “We do not, because the company must first demonstrate that all vaccines will be high quality“Added the deputy executive director of the EU regulatory body. AstraZeneca was the first pharmaceutical company sign a contract with the European Commission, for a total of 300 million doses, with an option to An additional 100 million.
[ad_2]