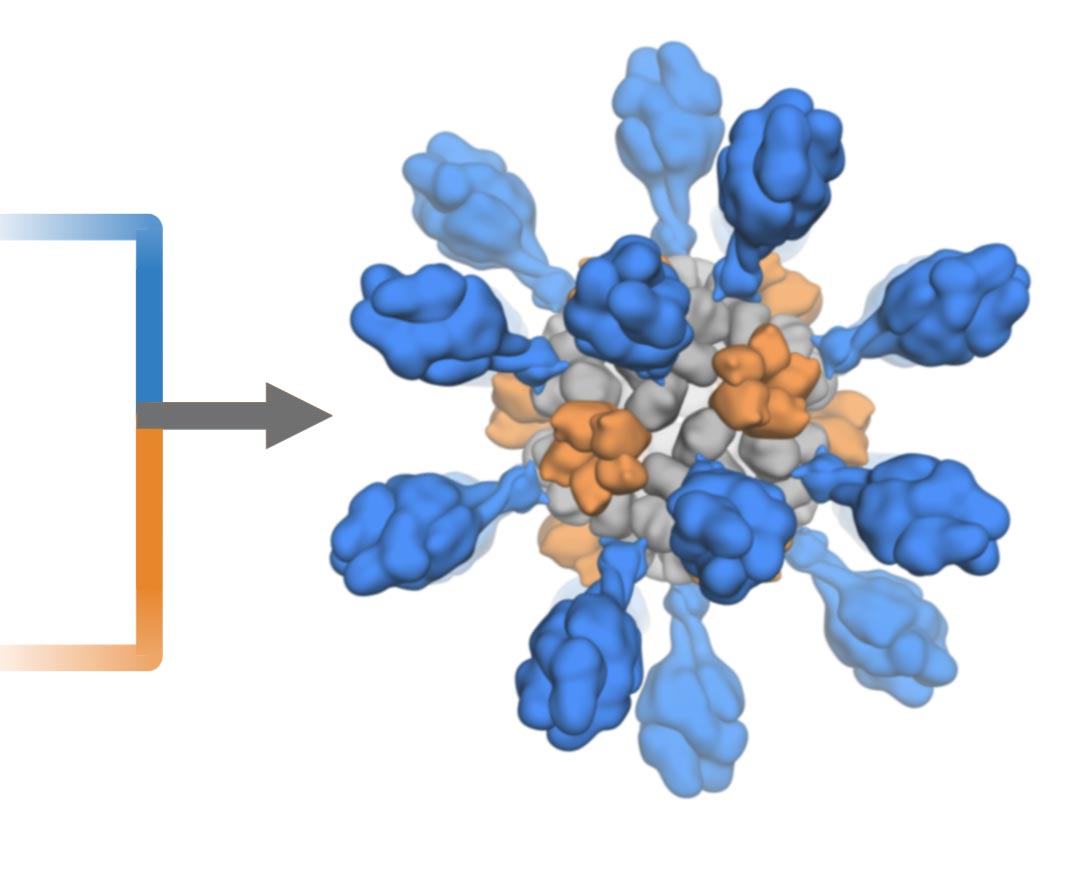

Representation of the artist’s ultrapotent COVID-19 vaccine candidate, in which 60 pieces of coronavirus protein (red) decorate nanoparticles (blue and white). The vaccine candidate was formulated using methods developed at the UW Medicine Institute for protein design. The molecular structure of the vaccine mimics the virus, which is responsible for its enhanced ability to provoke an immune response. Credit: Ian Hayden / UW Medicine Institute for Protein Design
Preclinical data published in the cell show that the nanoparticle vaccine stimulates very high levels of protective antibodies in the animal’s model dello.
Innovative nephrotic vaccine candidates for the epidemic coronavirus produce virus-neutralizing antibodies in rats that are found in more than 10 levels of people who have recovered. COVID-19 Infection. No. Designed by scientists And Washington Washington University The Seattle School of Medicine Medicine, a vaccine candidate, has been transferred to two companies for clinical development.
Compared to vaccination with soluble SARS-CoV-2 The spike protein, which is based on many of the leading COVID-19 vaccine candidates, the new nanoparticle vaccine produces 10 times more neutral antibodies in mice, even at six times lower doses. Data after immunization also show a strong response of B-cells, which may be crucial for immune memory and sustainable vaccine effect. When a single human primate is administered, the nanoparticle vaccine neutralizes antibodies targeting various sites on the spike protein. Researchers say these viruses can ensure protection against mutated strains, if they occur. Spike proteins are part of the coronavirus infectivity machinery.
The findings were published on October 30, 2020 Cell. The main authors of this paper are Alexandra Valls, David Wesler’s laboratory research scientist, UW associate professor of biochemistry; And Brooke Fiala, research scientist at Neil King’s Lab, UW Assistant Professor of Biochemistry.
The vaccine candidate was developed using structure-based vaccine design techniques discovered in UW Medicine. It is a self-assembling protein nanoparticle that displays 60 copies of the receptor-binding domain of the SARS-CoV-2 spike protein in a fairly immunogenic array. The molecular structure of the vaccine roughly mimics the virus, which is responsible for its enhanced ability to provoke an immune response.
“We hope our nanoparticle platform can help fight this epidemic that is causing so much damage to our world,” said King, inventor of the Institute for Computational Vaccine Design Technology at the Institute for Protein Design. “The strength, stability and productivity of this vaccine candidate sets it apart from many under investigation.”
Hundreds of candidates for the Covid-19 vaccine are in development worldwide. Many require large doses, complex production and cold-chain shipping and storage. An ultrapotent vaccine that is safe, effective at low doses, easy to produce outside the freezer and stable, can enable vaccination against COVID-19 globally.

Production schematic shows how coronavirus proteins are added to computer-designed nanoparticle platforms to create a candidate vaccine against covid-19. The vaccine candidate was created in animal models by researchers at The Washington School of Medicine. Credit: Ian Hayden / UW Medicine Institute for Protein Design
“I am delighted that our study of the antibody response to coronavirus has led to the development of this promising vaccine candidate,” said Weisler, who advocated the concept of a multivalent, receptor-binding, domain-based vaccine.
The lead vaccine candidate in this report is being given a non-special and royalty-free license during the epidemic by Washington University. The Seattle biotechnology company, co-founded in 2019 by a licensee, Icosavex, King, is currently pursuing studies to support regulatory filing and has begun good manufacturing practices of the U.S. Food and Drug Administration. To accelerate progress through Icosavex in the clinic, Amgen has agreed to create a key intermediate for this initial clinical study. Another licensee, S.K. Is advancing his studies to support bioscience, clinical and further development.
References: “SARS-Cavi-2 Alexandra C. V. L., Brooke Fiala, Alexandra Schaufer, Samuel Warren, Minh N. Fham, Michael Murphy, Longping v. Tse, Laila Shehata, Megan A. O’Connor, Chengbo Chen, Mary Jane Navarro, Marcos C. Miranda, Dileh Patty, Rashmi Ravichandran, John c. Kraft, Cassandra Ogohara, Ne Ne Pulsar, Sara Chalk, E-Chiang Lee, Catherine Guerrero, Elizabeth Capel, Cameron M. Chow, Claire Sideman, Edgar A. Hodge, Brian Brown, Jim T. Fuller, Kenneth H. Dinnon, III, Lisa e. Gralinski, Sarah R. List, center l. Gully, Thomas B. Lewis, Miklos Gutman, Helen Y. Chu, Kelly K. Lee, Deborah H. Fuller, Ralph S. Barrick, Paul Callum, Lure Ren Carter, Marion Pepper, Timothy P. Sheehan, David Weissler and Neil P. King, Accessed 26 October October 2020, Cell.
DOI: 10.1016 / j.sell.2020.10.043
The research reported in the cell was conducted by the National Institute of Allergy and Infectious Diseases (DP1AI158186, HHSN272201700059C, 3U01AI42001-02S1), National Institute General of General Medical Sciences (NGE). HDTRA1-18-1-0001), Pew Biomedical Scholars Award, Infectious Diseases Award from Burrows Welcome Fund in Investigators, Fast Grants, Animal Models Contract HHSN 272201700036 I-75N 93020 FROSONORO Relief funds, and gifts from the pair Green and Mike Halperin and from The Dacius Project.