/data/photo/2020/09/08/5f56feb8f3969.jpg)
[ad_1]
KOMPAS.com- The corona vaccine from various biotech companies from various countries that Indonesia has purchased will be distributed soon next month.
Quoted from the news Kompas.com, Monday (12/10/2020), according to a press release from the Ministry of Communications, the government is scheduled to be available in November 2020.
During a working visit and bilateral meeting with the Minister of Foreign Affairs and the ranks of the Government of China in Yunan, the Coordinating Minister of Maritime Affairs and Investments Luhut Pandjaitan together with the Minister of Health Terawan Agus Putranto and his entourage met with three producers of the corona vaccine, on Saturday (10/10/2020).
The three producers, namely CanSino, G42 or Sinopharm and Sinovac, are about to finalize the purchase of vaccines for Covid-19.
Also read: Making Corona vaccines threatens the lives of sharks, why?
President Joko Widodo has called for the Covid-19 vaccination program to be immediately released to the public. He asked the community to know the details of this program plan, so that it can run smoothly.
So will some of the corona vaccines currently in phase 3 clinical trials be effective if used next month?
In response to this, molecular biologist Professor Amin Soebandrio said that the use of vaccines in conditions like the current pandemic was immediately possible.
“However, in this case the POM Agency and the Ministry of Health must have given permission. Because the authority rests with these two institutions,” Prof. Amin said when contacted. Kompas.com, Monday (12/10/2020).
Also read: Why a clinical trial is needed for Corona vaccines in children, this is the pediatrician’s explanation
Currently, most of the vaccines are being clinically tested in Indonesia. Some of them are in the works.
The director of the Eijkman Institute for Molecular Biology (LBM) added that as long as the vaccines meet the requirements, it is possible to use them immediately.
Professor Amin explained that several countries issued the Emergency Use Authorization (USA), which is a permit for the emergency use of vaccines for Covid-19 due to various considerations.
“If it is published emergency use Therefore, vaccines can be used with various considerations related to the pandemic situation in question, “Amin explained.
This is because many people hope that the corona virus vaccine can be used immediately to overcome the current Covid-19 pandemic.
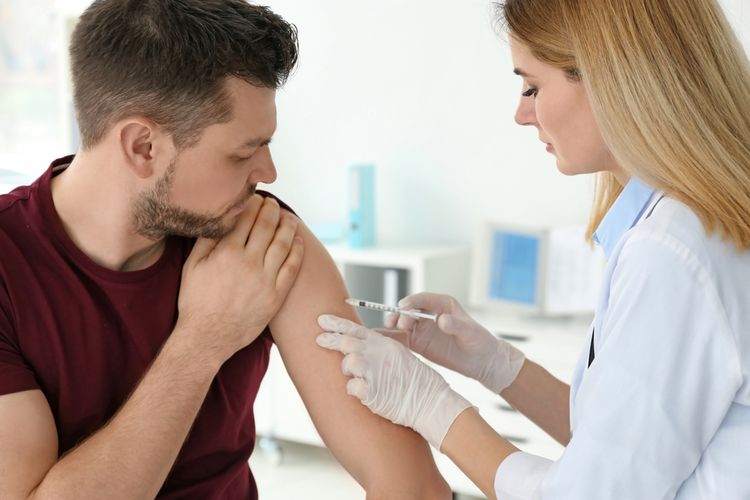 Illustration of the application of the corona vaccine, Covid-19 vaccination against the corona virus pandemic.
Illustration of the application of the corona vaccine, Covid-19 vaccination against the corona virus pandemic.
Although he does not know which vaccine will be used in November, Professor Amin emphasized that everything depends on the authority of the BPOM and the Ministry of Health.
Criteria for the use of emergency vaccines
Regarding the conditions that must be met so that the Covid-19 vaccination can be carried out immediately, Prof. Amin explained that the vaccine has completed phase 1 and phase 2.
Meanwhile, phase 3 should also be tested like phase 1 and phase two, but with a much larger number of populations or test subjects to demonstrate the efficacy of the vaccine being developed.
“Actually, there are considerations, but they are not necessarily used. For example, clinical trials have been 50 percent done and all show the same (successful) results. Nothing has failed. So that may be one of the considerations. that are given. emergency use“explained Prof. Amin.
Also read: British scientists inhaled the Corona vaccine
However, Prof. Amin recalled that clinical trials have yet to be completed, even though the EUA has been published. Or in other conditions, for example a clinical trial that has been only partially conducted, but the results are considered satisfactory.
However, when the situation is urgent and cannot be postponed, emergency use can be made. However, Prof. Amin explained that the use of emergency vaccines is only administered to certain populations.
“For certain groups, it cannot be used widely. One country is also implementing this by giving the military the Covid-19 vaccine,” Amin added.
Amid the massive clinical trial of the foreign-produced coronavirus vaccine for thousands of people in Indonesia, a domestic vaccine is also being developed.
Also read: Expert: We recommend you try the Corona Sinovac vaccine in the Red Zone, why?
Professor Amin said that the manufacture of a red and white vaccine to combat Covid-19, which is currently being carried out in Eijkman’s laboratory, is in the vaccine seed development stage.
“The phase is still in the laboratory phase, but it is already 55 percent. We are currently waiting for protein secretion,” Prof. Amin said.
The red and white vaccine in phase 1 of the clinical trial is expected to take place in the second quarter of next year.
“Meanwhile, all phases of the Indonesian corona vaccine clinical trial are expected to be completed by the end of next year (2021),” Prof. Amin said.