/data/photo/2020/08/15/5f379ba20b55e.jpg)
[ad_1]
KOMPAS.com – Rapid antigen testing becomes a new condition for travel by the government.
Those traveling out of town should prepare rapid antigen test results.
This rule takes effect from December 18, 2020 to January 8, 2021. Previously, trips could be made with negative results of the rapid antibody test.
In terms of price, the rapid antigen test is more expensive than the rapid antibody test.
Read also: [POPULER TREN] Different rapid test for antigen, antibody and PCR
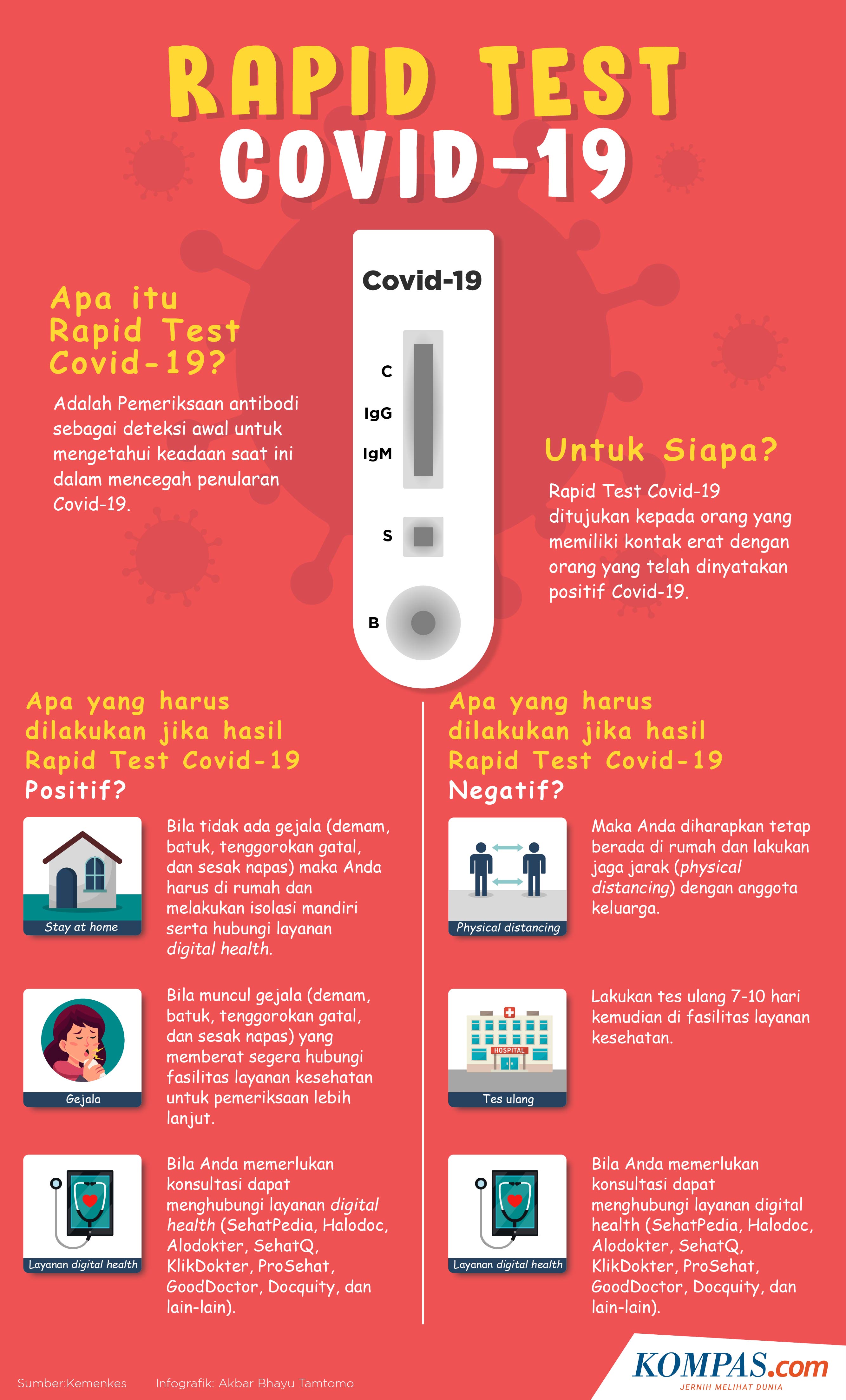
What do we need to know about rapid test antigen?
1. More accurate than the rapid antibody test
The rapid antigen test requires an oropharyngeal or nasopharyngeal swab sample. rapid antibody test using a blood sample.
A rapid antigen test is often called an antigen swab. This test is considered more accurate than the antibody test because it can identify the virus in nasal and throat secretions.
The inspection can be done in a location that has a biosafety cabinet.
Rapid antigen testing can be used to detect people under surveillance (ODP) and patients under surveillance (PDP) in areas without testing facilities. Reverse chain reaction of transcriptase-polyumerase (RT-PCR).
Rapid antigen test only when put on screen Initially, the results have yet to be confirmed by the RT-PCR test.
It is also said that the antigen test can detect the corona virus protein when the virus in a person’s body is at the most infectious level.
2. Prices vary
 A resident is undergoing an antigen smear at the NTB Provincial Health Laboratory, in an effort to prevent the spread of covid-19 in NTB.
A resident is undergoing an antigen smear at the NTB Provincial Health Laboratory, in an effort to prevent the spread of covid-19 in NTB.The World Health Organization (WHO) has approved the use of rapid emergency antigen tests in countries with low numbers of PCR tests.
WHO Director General Tedros Adhanom Ghebreyesus said the antigen test can produce results in 15-30 minutes.
In October, the WHO will distribute two antigen tests, the brand Abbott (United States) and SD Biosensor (South Korea) to several countries in cooperation with various institutions, such as the Bill & Melinda Gates Foundation.
Reported Kompas.com, Dec 16, 2020, the price of rapid Covid-19 antigen test in Indonesia still varies, in the range of IDR 349,000-IDR 665,000.
3. US Studies on Rapid Antigen Testing
According to a study published on November 2, 2020, the Louisiana Department of Health, United States, does not recommend rapid antigen testing for asymptomatic people who have never been exposed to patients with Covid-19.
Antigen tests, such as Abbott Laboratories’ BinaxNOW test, which looks for signs of viral proteins, can miss some infections, such as Covid-19.
The rapid antigen test is said to have the potential to give a false positive result.
Also Read: When Rapid Antigen Test Failed To Detect People Without Covid-19 Symptoms …
4. FDA Warning
Meanwhile, the United States Food and Drug Administration (FDA) has warned of a possible false positive result of a rapid antigen test to detect coronavirus infection, especially if the test was used incorrectly.
The FDA said it had received false positive reports from nursing homes and other health services.
According to the FDA, reading the test results, either before or after the time specified in the instructions, can show a false positive or negative.
This refers to the US antigen authorization provisions which stipulate that an authorized laboratory must follow the instructions for use regarding the administration of the test and the reading of the results.
Also, antigen tests that are not stored properly before use run the risk of giving false results.
Processing multiple samples at the same time can affect test results because it can be difficult to guarantee the correct incubation time for each sample.
“Take care to minimize the risk of cross contamination when testing patient samples, which can lead to false positive results,” the FDA said.
The FDA advises the Centers for Disease Control and Prevention (CDC) to recommend a protocol for antigen testing in nursing homes and to consider retesting to confirm results within 48 hours of testing. positive.
“In general, antigen tests are not as sensitive as molecular tests,” explains the FDA.
Also Read: FDA Warns Covid-19 Antigen Test Results May Be False Positive
Source: Kompas.com (Nur Fitriatus, Jawahir Gustav / Editor: Irawan Sapto Adhi, Gloria S, Sari H, Rizal S)
Infografik: Rapid Covid-19 Test