[ad_1]
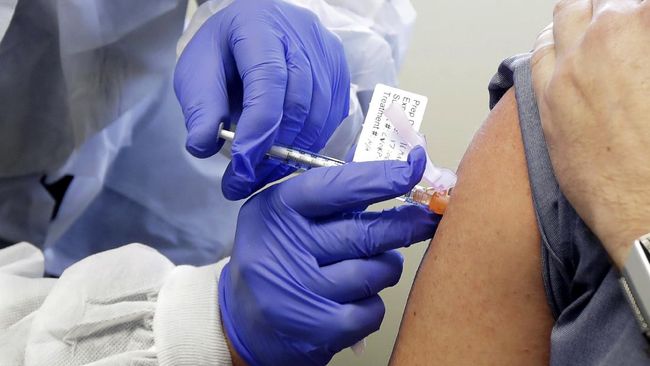
Jakarta, CNBC Indonesia – The latest news from Bandung comes from the Food and Drug Administration (BPOM), which continues to monitor clinical trials of the Sinovac vaccine. More than 1,600 volunteers are registered to participate in clinical trials conducted in Bandung.
The spokesperson and chairman of the Covid-19 Task Force Expert Team, Wiku Adisasmito, said that until now there were no reports related to the phase three clinical trial of the Chinese vaccine, Sinovac, developed by Biofarma.
“There is no effect of the reports on clinical trials, and the main role of the Indonesian government is to ensure the availability of vaccines for Indonesian citizens to achieve immunity,” he said during a virtual press conference in Jakarta on Wednesday (10 / 7/2020).
He also added that Indonesia would be selective in the choice of vaccines, including Sinopharm and other pharmaceutical companies. This is to ensure that vaccines entering Indonesia are safe, which is reflected in the data and research results.
“In line with that, the multilateral cooperation of the Indonesian government also participates in the development of domestic vaccines, such as the development of the red and white vaccine by Eijkman and the Ministry of Research and Technology,” he concluded.
On another occasion, the chairman of the Covid-19 vaccine clinical trial research team, Professor Kusnadi Rusmil, said his group had already given the first injection to 1,320 volunteers and the second to 660 volunteers.
“It looks like all the injections will be completed next week. We just have to follow them for 6 months,” he said.
Kusnandi Rusmil revealed that the concern in this phase three clinical trial was safety, efficacy and efficiency. Safety in monitoring local reactions, if there is swelling, redness of the skin, etc. One more systemic reaction, like that experienced by the volunteers, pain, body heat and others.
Clinical trials are expected to be completed by the end of the year and can be used starting in March 2021. This vaccine will be prioritized for healthcare workers, workers, children and other productive workers to keep the economy going.
“The Sinovac vaccine is also being tested in Brazil, India, Bangladesh and Turkey. We will see how the results are going to be in other countries. If this vaccine is sold freely, it should have the same result. After that WHO approval. Yes WHO says it’s good, it can be sold globally, “he said.
One of the 1,620 volunteers who participated in the phase 3 trial at Padjajaran University in Bandung some time ago was the Governor of West Java, Ridwan Kamil. He himself admitted that, although he was part of the government that ran it directly, he volunteered to be one of the 1,620 volunteers for the Covid-19 vaccine.
Ridwan Kamil assured the public that the vaccine clinical trial conducted at Padjajaran University in Bandung went through three phases of clinical trials some time ago and was witnessed by President Joko Widodo.
“The phase one vaccine is injected into volunteers whose number is less than 100 people. Phase two is injected into volunteers with a number between 100 to 1000 people. And phase three is for volunteers greater than 1000 people and exactly 1,620 volunteers, “shared his story in an interview conducted by Juru. Speaking about the Covid-19 Management Working Group of Dr. Reisa Brotoasmoro, which was broadcast on the YouTube channel of the Presidential Secretariat, on Friday (09/10/2020).
His experience undergoing trial required that he attend 5 visits. First run PCR tests, rapid tests and the like for conditioning. The second visit, receiving the injection of the first phase vaccine, the third visit the second phase injection, the fourth and the fifth visits to the blood draw to check the reaction of the injected vaccine.
“After being injected with the vaccine, the antibodies in my body are abundant or not. Now if the abundance reaches 90%, it means that my body is ready to fight the Covid-19 virus that will attack my body. The second blood draw will be done in December 2020 and to see the results. , “he said. So if the December blood test results are successful, then the mass production of vaccines can only be started and mass vaccination continues.
In fact, he said the government’s efforts were not easy. And there are still groups in society who doubt the safety of vaccines. Even Ridwan, who followed the voluntary vaccination process, was accused of only faking or spreading deception (fake news), when his photo during the blood collection process was uploaded to his personal social media account and circulated widely on social media. .
“The public perception, people who do not understand think I am lying. Because according to those who do not understand, the syringe is still like the old model, whereas in the vaccine test a modern syringe called a vacutainer is used,” he said.
[Gambas:Video CNBC](hps / hps)